41 what type of reaction is alka seltzer and water
In my hypothesis, I stated that if rate of reaction is related to the surface area of a reactant, then crushing a tablet of alka seltzer would result in a higher reaction rate. After completing the experiment, it was clear that changing the amount of surface area of the alka seltzer drastically improved the rate of reaction, by almost 35%. The gas (carbon dioxide) formed when the Alka-Seltzer® tablet dissolves builds up pressure inside the sealed container until the enclosed area cannot contain it The point of least resistance will pop. 20 min. Adding VARIABLES: Re-try the experiment with another Alka-Seltzer® tablet, using a 1/2 tablet and then a full size tablet.
Alka Seltzer tablets endure a chemical reaction changing from a solid to a liquid state when placed in water. The temperature of the water determines how fast the tablet completely dissolves within...

What type of reaction is alka seltzer and water
Carbon dioxide, water, and sodium citrate solution Explanation: Alka Seltzer is a mixture of sodium bicarbonate, citric acid, and acetyl salicylic acid. The sodium bicarbonate and citric acid are there mainly to create a pleasant tasting fizzy antacid solution. What happens when Alka-Seltzer is added to water? drop the tablet in water, they mix and react, forming carbon dioxide gas which bubbles up to the surface of the water. If the water and Alka-seltzer are in a tightly sealed container, the gas will expand rapidly. What type of change occurred when adding Alka-Seltzer to water? May 13, 2019 · What happens when Alka Seltzer tablets are put in water? As an Alka-Seltzer tablet dissolves in water, it liberates carbon dioxide. The carbon dioxide dissolves in the water and then comes out of solution as a gas. This carbon dioxide gas has mass, but since it is a gas it escapes from the container and diffuses into the atmosphere.
What type of reaction is alka seltzer and water. • Extra: Test Alka-Seltzer tablets in a wider range of temperatures, and then draw a graph showing the time it takes a tablet to dissolve in water at each temperature (check with a thermometer). Alka Seltzer tablets easily dissolve in water. Water releases hydrogen ions that can react with the base inside the Alka Seltzer tablet, sodium bicarbonate. When the sodium bicarbonate reacts with the hydrogen ions, carbon dioxide forms, which causes the bubbles you see when you drop an Alka Seltzer tablet in water. When an Alka-Seltzer tablet comes in contact with water, the tablet makes carbon dioxide gas through a chemical reaction (Image credit: by Ebarella_R, via Flickr). Alka-Seltzer is a medical drug that works as a pain reliever and an antacid (antacids help neutralize stomach acidity, such as heartburn). Dec 21, 2021 · When they enter water, the chemicals are released and can react. In Alka-Seltzer, the citric acid mixes with the base, bicarbonate, to form carbon dioxide bubbles. How quickly these bubbles form ...
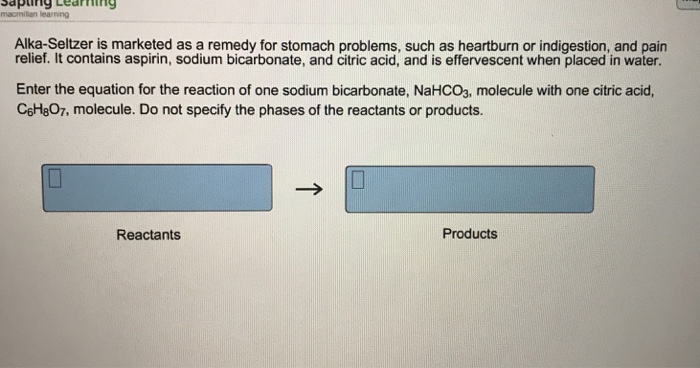
Alka-Seltzer is something that when it gets put into water it has a chemical reaction because it starts to bubble up and when it starts to bubble up the bubbles are carbon dioxide gas. Chemical reactions are one of the primary focuses for Chemical Engineers. From synthesizing polymers to treating water to creating fertilizers, chemical reactions are important in nearly every aspect of daily life. One job of Chemical Engineers is to classify, understand, and control these reactions to speed them up or slow them down. Chemical reactions occur when bonds within molecules are broken or formed. There are several things that signify that a chemical reaction took place. These include a change in color, the production of a gas or solid, and of course a change in chemical composition. The starting chemicals before a reaction are called the reactants, and the chemicals that are produced are called the products. The reaction in this activity involves using sodium bicarbonate and citric acid to produce water and carbon dioxide. Reaction: HCO3– (aq) + H+ (aq) → H2O (l) + CO2 (g) The tablets contain sodium bicarbonate (NaHCO3) and citric acid. When the tablet is dissolved in wa... Alka-Seltzer reacts with an acid to make carbon dioxide gas. When you added the tablet to the vinegar or water, you heard fizzing and saw bubbling. If the reaction is going faster, then it will take less time to finish and will fizz more vigorously. The speed of a reaction depends on the number of collisions between molecules. • Alka-seltzer tablets contain citric acid (an acid) and sodium bicarbonate (a base.) When you drop the tablet in water, they mix and react, forming carbon dioxide gas which bubbles up to the surface of the water. If the water and Alka-seltzer are in a tightly sealed container, the gas will expand rapidly.
Alka-Seltzer is a mixture of Aspirin (acetyl salicylic acid), anhydrous citric acid and sodium bicarbonate. Upon mixing in water, it liberates CO2 creating effevescence. Aspirin will help to relieve headache or muscle pain ( regular analgesic effect) while the bicarbonate buffer will neutralise excess acid. Not sure how Alka-Seltzer makes bubbles. My best guess would be CO2 gas bubbles. They could have a mixture of sodium bicarbonate separated by some type of dry acid on the other side. When it is dropped in water, the dry acid would react with the NaCO3. NaCO3 + HCl + H2O -> CO2 (gas) + NaCl (table salt) + H2O (did not bother to balance the equation) As an Alka-Seltzer tablet dissolves in water, it liberates carbon dioxide. The carbon dioxide dissolves in the water and then comes out of solution as a gas. This carbon dioxide gas has mass, but since it is a gas it escapes from the container and diffuses into the atmosphere. What type of reaction is Alka Seltzer? Chemistry Demo of Alka-Seltzer and Water
1. describe the chemical change that occurs when Alka-Seltzer® and water react in terms of properties of reactants and products. 2. use evidence to describe the law of conservation of matter. 3. determine at least two conditions that affect the reaction rate of Alka-Seltzer® tablets in water. 4. devise a method to measure reaction rate. 5.
Alka seltzer is a quick over-the-counter cure for an upset stomach. It is made of a base called bicarbonate. Bases are substances that can accept hydrogen ions, and acids are substances that can ...
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features Press Copyright Contact us Creators ...
The chemical reaction that causes the bubbling comes from two different compounds within the Alka-Seltzer tablet itself. The compounds do not react with each other until they dissolve in water.
Ultimately, the temperature has little effect of the reaction of water and Alka-Seltzer. However, warmer water will make the reaction occur and finish a bit faster.
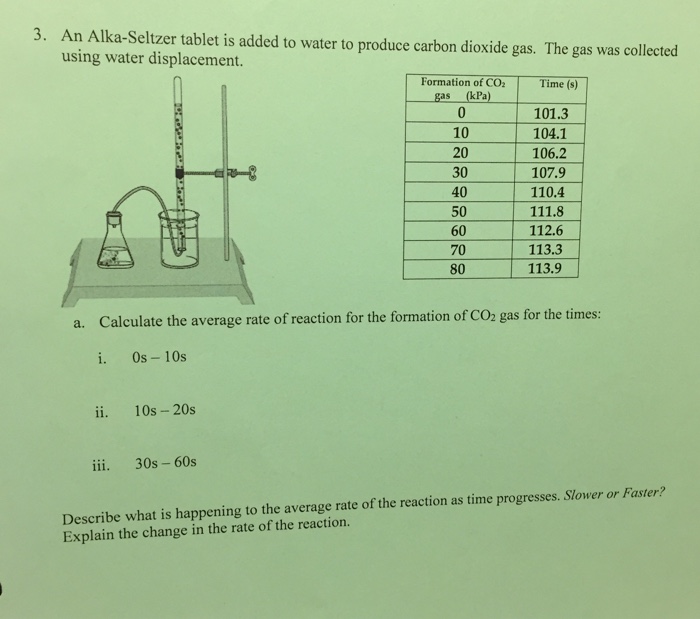
Part A. Rate of Reaction at Normal Pressure. STEP 1 Fill a 16 x 150 mm test tube 1/2 full of water. The water should be at about room temperature. STEP 2 Break an Alka-Seltzer tablet in half and drop the pieces into the test tube. STEP 3 Measure the time required for the reaction between the Alka-Seltzer and water to be completed. Record the time.
Purpose: Try to have a chemical reaction between Alka seltzer and water be controlled so that the lid of the canister pops of in exactly 15 sec. Hypothesis: If a 1/4 of a solid Alka-Seltzer tablet and 15 mL of hot water is added then the lid of the film canister will pop off in exactly 15 seconds. Introduction: v Temperature- causes atoms to move faster v Surface area- more space to react in v ...
In the reaction of the Alka-Seltzer tablet with water, one can make the assumption that the rate of the reaction is first-order. The rate of the reaction can be measured by observing the time (tx) taken for the Alka-Seltzer tablet to disappear. We can also assume that the rate constant for the process is proportional to 1/tx.
May 13, 2019 · What happens when Alka Seltzer tablets are put in water? As an Alka-Seltzer tablet dissolves in water, it liberates carbon dioxide. The carbon dioxide dissolves in the water and then comes out of solution as a gas. This carbon dioxide gas has mass, but since it is a gas it escapes from the container and diffuses into the atmosphere.
What happens when Alka-Seltzer is added to water? drop the tablet in water, they mix and react, forming carbon dioxide gas which bubbles up to the surface of the water. If the water and Alka-seltzer are in a tightly sealed container, the gas will expand rapidly. What type of change occurred when adding Alka-Seltzer to water?
Carbon dioxide, water, and sodium citrate solution Explanation: Alka Seltzer is a mixture of sodium bicarbonate, citric acid, and acetyl salicylic acid. The sodium bicarbonate and citric acid are there mainly to create a pleasant tasting fizzy antacid solution.
































0 Response to "41 what type of reaction is alka seltzer and water"
Post a Comment