42 how does sugar affect the surface tension of water
How does adding salt affect the surface tension of water ... Yes adding salt to water does increase the surface tension of water although not by any significant amount. It is a very common misconception that salt is a surfactant i.e. a compound that either lowers or breaks surface tension. However experiments done with salt water show that surface tension actually increases when salt is added to pure water. Surface Tension - water, pepper, detergent & sugar - YouTube Deane and Rob demonstrate the effects of pepper, detergent and sugar on the surface tension of water.
Biology 1: How Is Surface Tension of Water Affected By ... What if the experimental question was "How does sugar affect the surface tension of water?" Describe how you would answer this question using the Scientific Method. This question could be answered by following the same steps but with water that had sugar dissolved in it instead of soapy water.
How does sugar affect the surface tension of water
how does sugar affect the surface tension of water using ... Sep 30, 2018 · The surface tension in water is due to the hydrogen bonds in it. When sugar is added to it, the dissolution process occurs which leads to the breaking of the hydrogen bonds in water by the hydration energy generated by the interaction of sugar and water. Therefore sugar reduces the surface tension in water. what is the leading cause of surface tension in water ... The high surface tension of water is caused by strong molecular interactions. The surface tension arises due to cohesive interactions between the molecules in the liquid. At the bulk of the liquid, the molecules have neighboring molecules on each side.Aug 4, 2020. how does sugar affect the surface tension of water ... Oct 03, 2017 · When sugar is added to water ,the solute concentration of the water increases. The hydration energy is generated from the chemical reactions between sugar and water molecules. The hydration energy lowers the H-bonding (reduces the surface tension). Hence sugar lowers the surface tension Advertisement Survey Did this page answer your question?
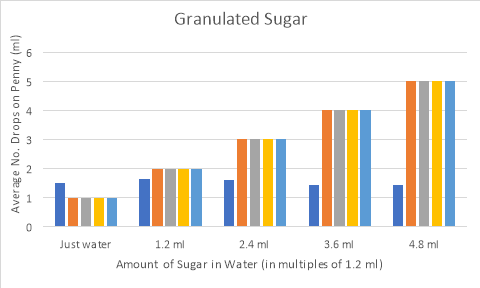
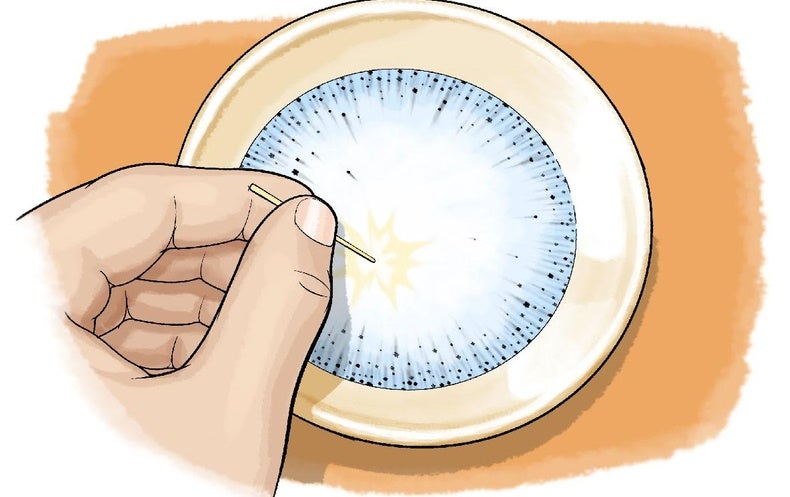
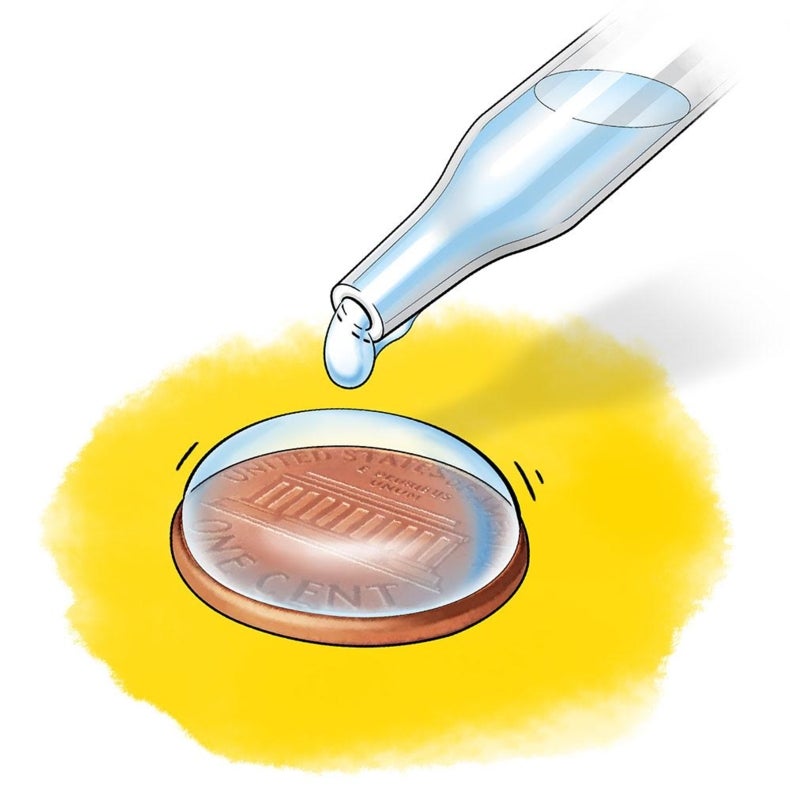
How does sugar affect the surface tension of water. PDF How is the Surface Tension of Water Affected By Soap? How is the Surface Tension of Water Affected By Soap? Introduction: Surface tension refers to water's ability to "stick to itself". Surface tension can be measured and observed by dropping water (drop by drop) onto a penny. The number of water drops that can fit on a penny will surprise you. 1. Surface Tension and Water | U.S. Geological Survey Why bubbles are round: The surface tension of water provides the necessary wall tension for the formation of bubbles with water. The tendency to minimize that wall tension pulls the bubbles into spherical shapes. Surface tension and droplets: Surface tension is responsible for the shape of liquid droplets. "How does sugar affect the surface tension of water? Find the quality of the following two phase liquidvapor mixture a Water at 40oC and specific volume 10 m3 kg b Propane at 20 bar and specific internal energy 300 kJkg c Refrigerant 134a at 90 lbfin2 and specific enthalpy 90 Btulb d Ammonia at 20oF and specific volume 11 ft3 lb ... Do polysaccharides such as dextran and their ... - PubMed It has been reported in the literature that sugars such as dextrose and sucrose increase the surface tension of water. The effect was interpreted as a depletion of the solute molecules from the water-air interface.
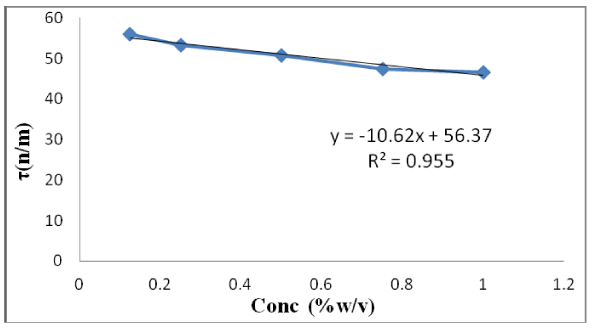
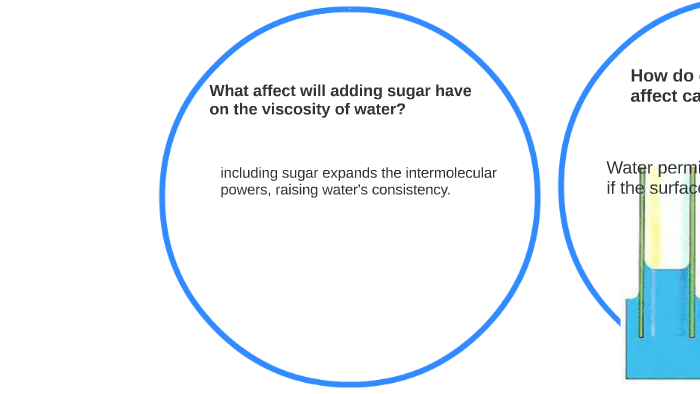
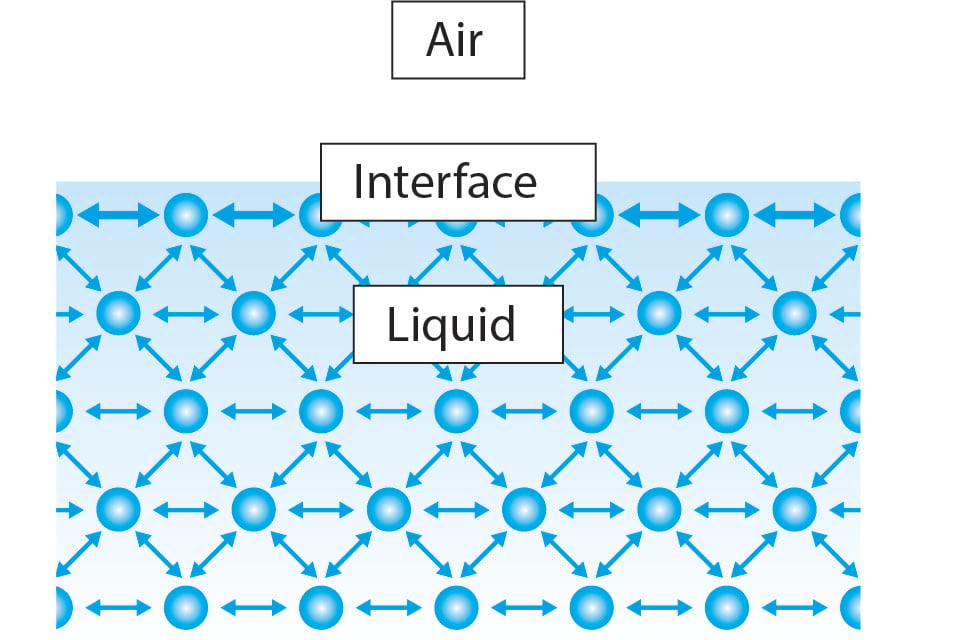
How does changing the sugar concentration in water affect ... Answer (1 of 4): Water has a viscosity, a resistance to shear, due to the fact that water molecules have some attraction for each other, due to the polarity of the molecules, The protons, bound to their own oxygen, still have some attraction to other oxygens. Gases, with little attraction to othe... Surface Properties of Sugar Solutions - Confex The results show that the effect of sugar on the surface tension of water is more complicated than the unsubstantiated interpretation reported in the literature. The surface tension of sucrose solutions at low concentrations (i.e. up to 5 wt.%) is near that of water, but as the concentration increases the surface tension increases even above ... How Does Soap Affect The Surface Tension Of Water ... First we would make an observation about how water's surface tension could be affected. Next we would propose a hypothesis. Like if we add sugar to water the water's surface tension will be greater. Then we would follow the steps of the experiment performed earlier. We would then be able to prove or disprove the hypothesis we created. How does surface tension affect water? - AskingLot.com How does surface tension affect water? Surface tension is the tendency of liquid surfaces to shrink into the minimum surface area possible. Because of the relatively high attraction of water molecules to each other through a web of hydrogen bonds, water has a higher surface tension (72.8 millinewtons per meter at 20 °C) than most other liquids.
What if the experimental question was "How does sugar ... Ali Abdullah Hi There, The surface tension in water is due to the hydrogen bonds in it.When sugar is added to it,the dissolution process occurs which leads to the breaking of the hydrogen bonds in water by the hydration energy generated by the interaction of sugar and water.Therefore sugar reduces the surface tension in water. Thanks How Does Soap Affect Water Surface Tension? - BehindTheWash The answer is yes. Merely touching the water mixture itself can break the cohesion and tension on the surface. For example, if you place a needle on top of a glass filled with water, it will just sit there because of the tension in the water. However, if you push the needle down into the glass, the force exerted will effectively drop the object ... Why does sugar affect the surface tension in water? - Answers Nov 02, 2010 · Why does sugar affect the surface tension in water? - Answers The surface tension of water is due to the hydrogen bonds contained in it.When sugar is added to it,the dissolution process occurs... UCSB Science Line However, dissolving salts in water typically increases surface tension, and dissolving sugar in water has no effect. Answer 2: Van der Walls forces influence surface tension, as well as many other properties, such as boiling point or melting point. In general, if there are stronger intermolecular forces, the molecules tend to hold together more ...
Does salt water increase surface tension? - R4 DN It has been reported in the literature that sugars such as dextrose and sucrose increase the surface tension of water. The effect was interpreted as a depletion of the solute molecules from the water-air interface. When the common salt is dissolved in pure water the surface tension?
everyday chemistry - Salt and sugar surfactants ... The surface tension of water is increased by the dissolution of either glucose ( C X 6 H X 12 O X 6) or N a C l. The following are excerpts from this table: Solution Temp ( o C) Surface Tension (mN/m) Water 25 71.97 Water 0 75.46 6M aqueous N a C l 20 82.55 Sucrose (55%) + water 20 76.45
Is there a way to increase the surface tension of water The effect of adding an unrelated chemical to a substance, and thereby changing its surface tension, is demonstrated by the example of putting soap (a surfactant) in water to reduce the surface ...
PDF How is the Surface Tension of Water Affected By Soap? 10. What if the experimental question was "How does sugar affect the surface tension of water?" Describe how you would answer this question using the scientific method. If you have time, you can test this. RERUN Conclusion: Analyze the data and draw conclusions. Write a paragraph below (using complete sentences) that explains how soap affects ...
How does the purity of water affect surface tension? - Answers The surface tension of water is due to the hydrogen bonds contained in it.When sugar is added to it,the dissolution process occurs which leads to the breaking of the hydrogen bonds in water by the...
Lab: How is the Surface Tension of Water Affected By Soap ... Introduction: Surface tension refers to water's ability to "stick to itself", due to the forces of cohesion. Surface tension can be measured and observed by dropping water (drop by drop) onto a penny. The number of water drops that can fit on a penny will surprise you. Initial Observation: Observe surface tension by seeing how
how to increase surface tension of water - Lisbdnet.com It has been reported in the literature that sugars such as dextrose and sucrose increase the surface tension of water. The effect was interpreted as a depletion of the solute molecules from the water-air interface. What causes high surface tension in water? The high surface tension of water is caused by strong molecular interactions.
water - How does NaCl (or any inorganic salt) increase ... See: Does NaCl reduce the surface tension of water? Or is the compound distributed evenly and because the ionic bonds are stronger than the attractions between water molecules, the surface tension increases? Read here a reddit answer to why do ionic compounds such as NaCl increase the surface tension of water? Explain like I'm 5 please.
how does sugar affect the surface tension of water ... Oct 03, 2017 · When sugar is added to water ,the solute concentration of the water increases. The hydration energy is generated from the chemical reactions between sugar and water molecules. The hydration energy lowers the H-bonding (reduces the surface tension). Hence sugar lowers the surface tension Advertisement Survey Did this page answer your question?
what is the leading cause of surface tension in water ... The high surface tension of water is caused by strong molecular interactions. The surface tension arises due to cohesive interactions between the molecules in the liquid. At the bulk of the liquid, the molecules have neighboring molecules on each side.Aug 4, 2020.
how does sugar affect the surface tension of water using ... Sep 30, 2018 · The surface tension in water is due to the hydrogen bonds in it. When sugar is added to it, the dissolution process occurs which leads to the breaking of the hydrogen bonds in water by the hydration energy generated by the interaction of sugar and water. Therefore sugar reduces the surface tension in water.
























0 Response to "42 how does sugar affect the surface tension of water"
Post a Comment