40 difference between ionic and covalent
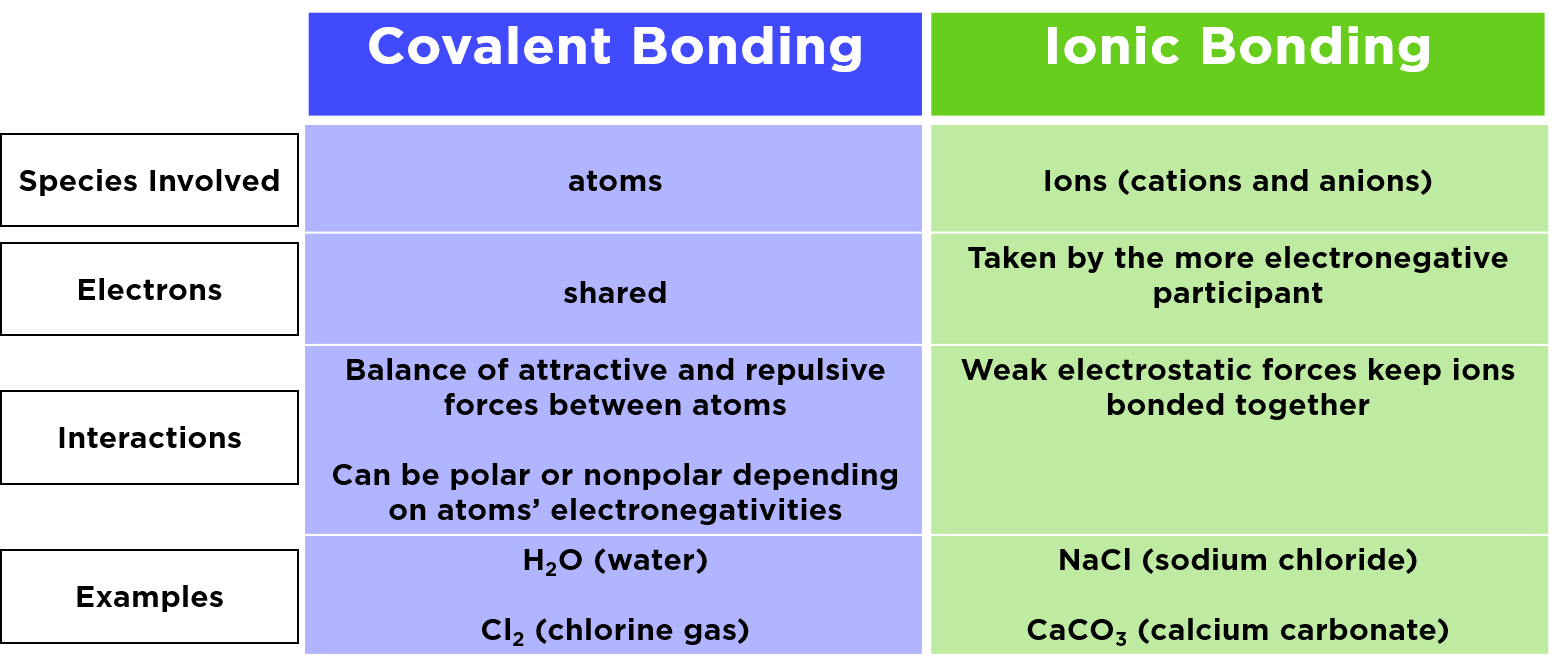
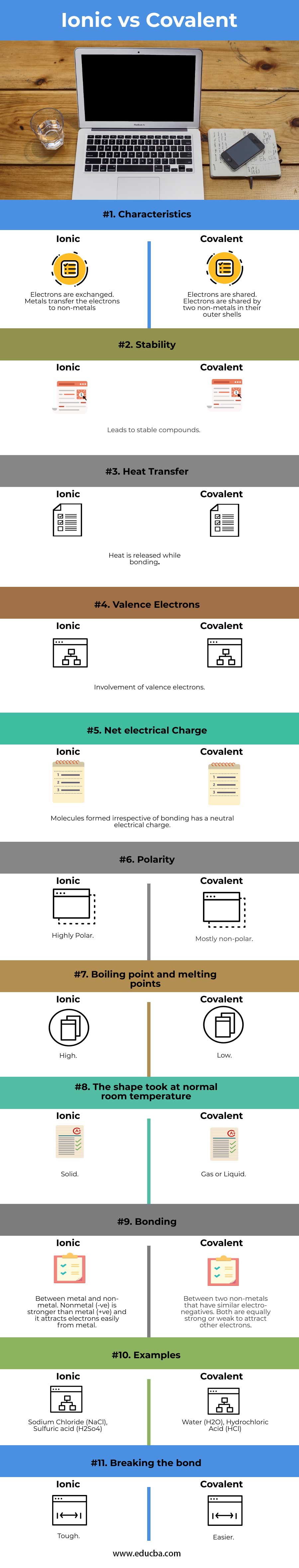
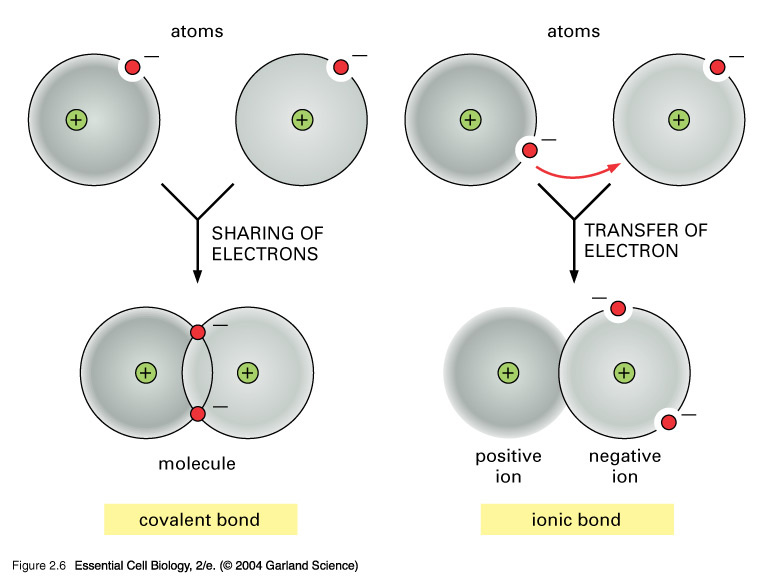
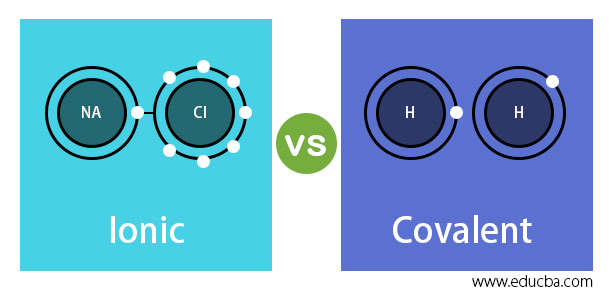
Ionic vs Covalent Bonds - Understand the Difference The two main types of chemical bonds are ionic and covalent bonds. An ionic bond essentially donates an electron to the other atom participating in the bond, while electrons in a covalent bond are shared equally between the atoms. The only pure covalent bonds occur between identical atoms. What is the difference between ionic and covalent crystals ... 1. Ionic bonds result from transfer of electrons, whereas covalent bonds are formed by sharing. 2. Ionic bonds are electrostatic in nature, resulting from that attraction of positive and negative ions that result from the electron transfer process; charge separation between covalently bonded atoms is less extreme.
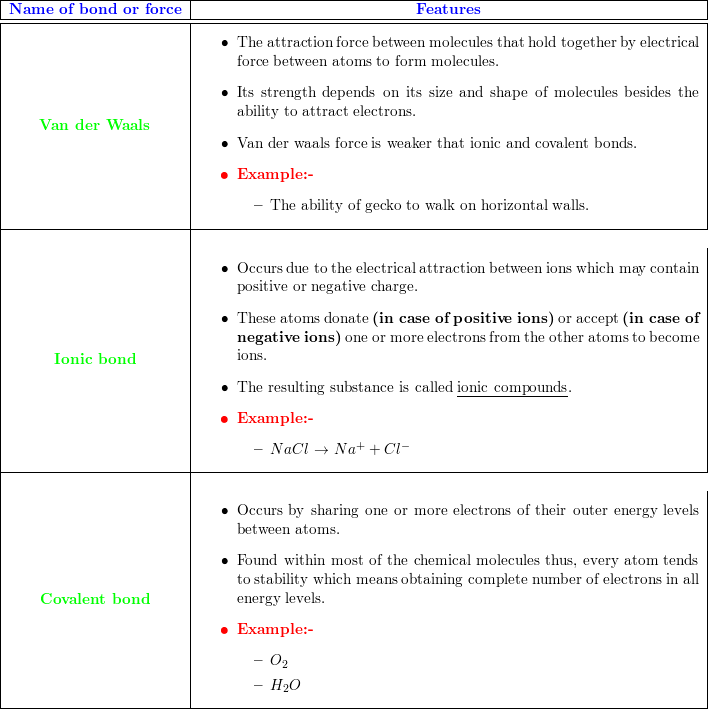
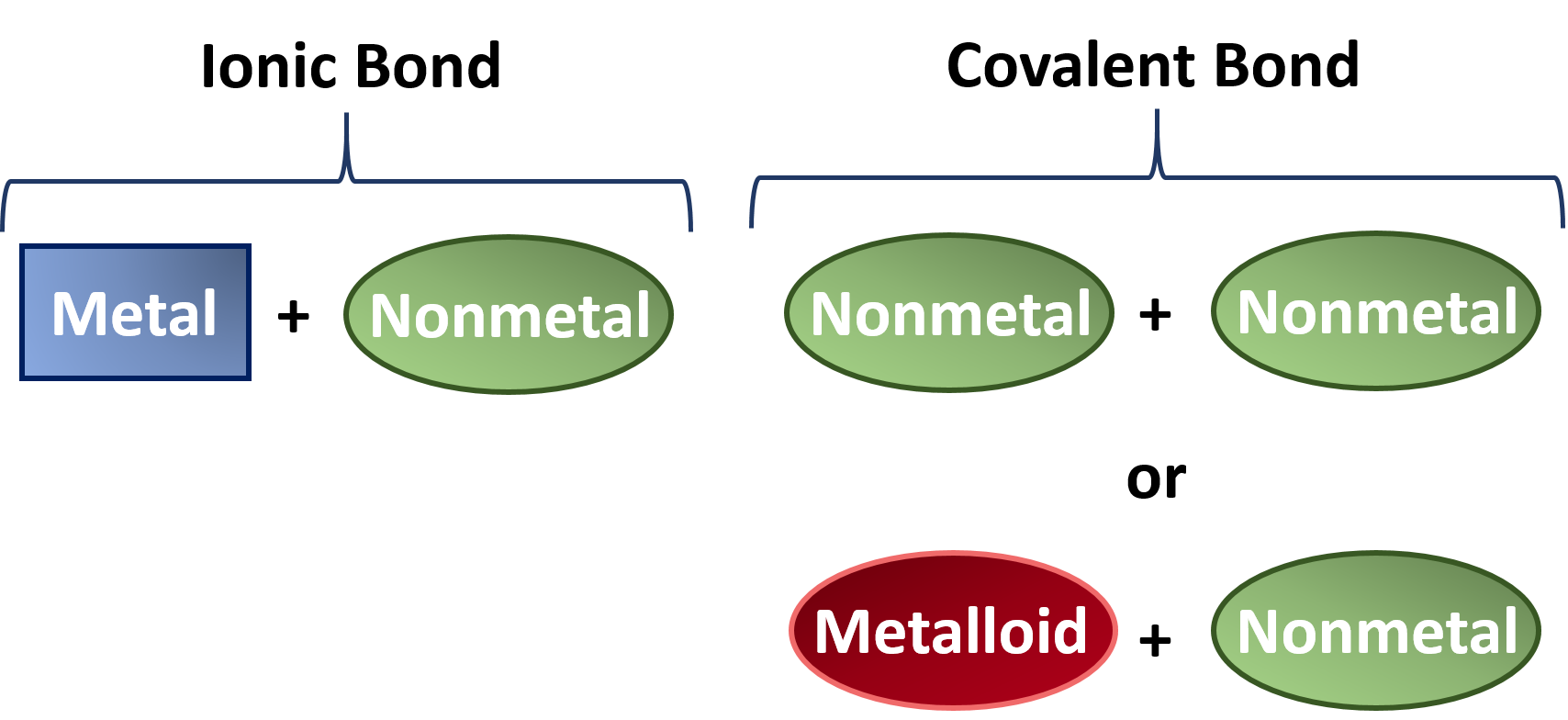
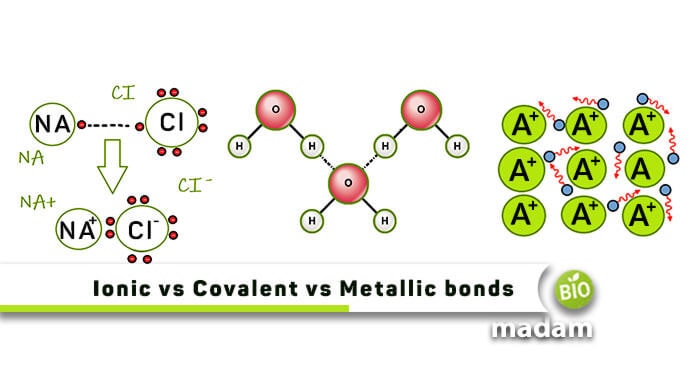
Difference Between Ionic, Covalent and Metallic bonds - Byjus Ionic bond The ionic bond is the electrostatic force of attraction between two oppositely charged ions. Ionic bonds join metals to non-metals. Covalent bond The covalent bond is also called a shared bond. These bonds join non-metals to non-metals. What are Metallic bonds? Metallic bonds are the chemical bonds that join metals to metals.

Difference between ionic and covalent
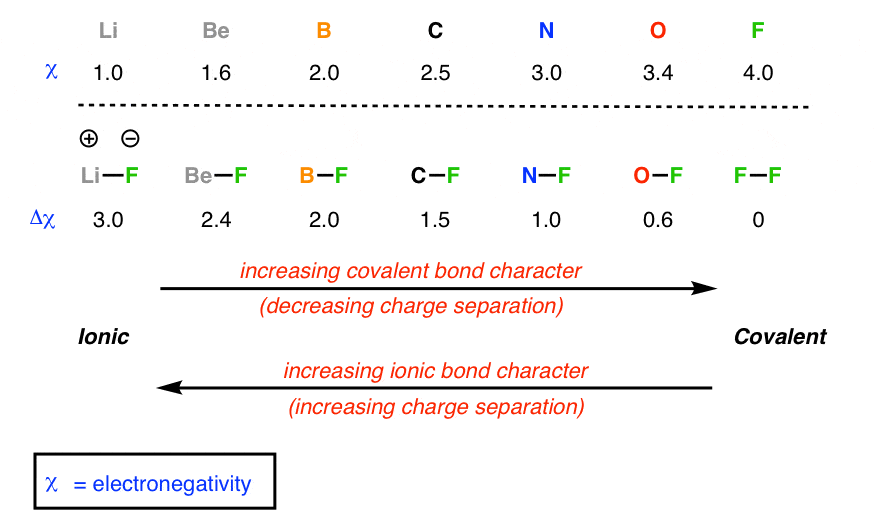
What is Difference between an ionic bond and a covalent bond? Difference between an ionic bond and a covalent bond Ionic bonding occurs when two atoms have an electronegativity difference greater than 1.7. One of the atoms takes one of the electrons from the outer shell of the other atom, that is, the metallic ion loses an electron, and the non-metallic ion gains the electron. What's the difference between ionic and covalent bonds - ZME ... Differences between ionic and covalent bonds. Covalent bonds are much more common in organic chemistry than ionic bonds. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms ... Main Differences Between Ionic and Covalent Bonds - Examples Since covalent bonds are just sharing their stuff, they aren't as solid of a bond as ionic bonds. You'll also see these as a gas or liquid rather than a solid like ionic bonds. But while covalent bonds might be a little weaker than ionic bonds, they can form between the same elements. The more you know. Difference Between Ionic and Covalent Bonds
Difference between ionic and covalent. Ionic or Covalent Simulation-4 (1).docx - Ionic or ... Ionic or Covalent? 1. Describe the difference between an atom and a molecule: Atoms are a single neutral particle.Molecules are neutral particles made of two or more atoms bonded together 2. Where are metal atoms located on the periodic table? Answered: Describe the difference between… | bartleby Question. Transcribed Image Text: Describe the difference between solvation of ionic compounds and solvation of covalent compounds, For each type of compound: • describe what forces hold the compound together • describe how and why they dissociate (come apart) in water. A- I U x2 X2 用 #3 X Next page us page MacBook Air 吕口 F3 F4 F2 ... Ionic And Covalent Bonds Review Sheet Answers Which Bond Is Stronger Ionic Or Covalent - Criteria and 23.11.2021 · Common chemical bonds include ionic, covalent, polar covalent, and metallic. Learn why and how these bonds form, and then apply and test that knowledge through a short quiz. Difference Between Ionic, Covalent and Metallic bonds with Start studying Bio 112 Final Review Ch 1-5. Difference Between Ionic and Covalent bond | Difference ... Covalent bonding takes place when the atoms occurs because the atoms in the compound have a similar ability to gain and lose ions. So, ionic bonds may form between metals and nonmetals while, covalent bonds form between two nonmetals. Ionic bonds can also be dissolved in water and other types of polar solvents.
PDF List the four major differences between ionic and covalent ... List the four major differences between ionic and covalent compounds. 1. Ionic bonds result from transfer of electrons, whereas covalent bonds are formed by sharing. 2. Ionic bonds are electrostatic in nature, resulting from that attraction of positive and negative ions that result from the electron transfer process; charge separation between ... Difference Between Ionic and Covalent (With Table) - Ask ... Main Differences Between Ionic and Covalent Ionic bond takes birth due to the transfer of electrons, while Covalent takes birth due to sharing of electrons. Ionic can conduct electricity while Covalent cannot do so. Ionic takes place between metals and nonmetals having opposite charges, while Covalent takes place between nonmetals only. Ionic vs Covalent - Easy Hard Science So what is the difference between ionic and covalent bonds? You could say that covalent compounds form individual structures of atoms, whereas ionic compounds are based on a repeating pattern of atoms. What? Let's look at some examples to show the difference between ionic and molecular (another word for covalent) substances. Difference between Ionic and Covalent Bond | Ionic vs ... Key difference: An ionic bond is a chemical bond between two dissimilar (i.e. a metal and a non-metal) atoms in which one atom gives up an electron to another. A covalent bond is another strong chemical bond. It takes place been similar atoms (i.e. two non-metals).
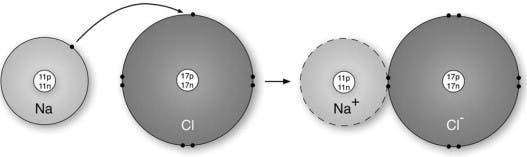
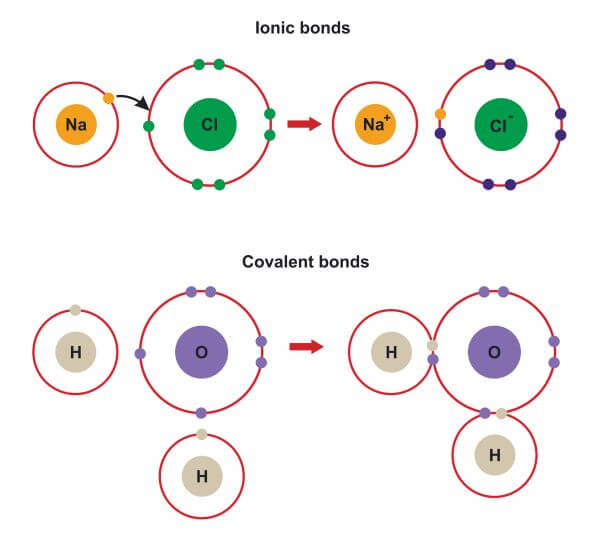
Ionic vs Covalent | Learn the Difference between Ionic and ... This bond is formed when two bonded atoms having opposite charges & varying electronegativity values, exchange electrons among them i.e. one atom transfers an electron to the other atom and the exchanged electron spends its time with the other atom. The atom that gave electron will have more protons and it will have a positive charge whereas the atom that gains an electron will have a negative charge. These types of bonding are called polar bond and in this compound, ions that lose electrons are called cations (metals) and the other ions that gain electrons are called anions (non-metals) and the compound neutralizes the positive charges of cations with negative gains of anions. An example of an ionic bond is chemical compounding between Sodium and Chloride that makes common salt NaCl. Sodium and Chloride having different electronegativity values when bonded will acquire a tendency to dissociate into ions in water. Sodium has 11 electrons and 11 protons and only one electron in outer... Difference Between Ionic and Covalent Bonds Flashcards ... What is the difference between ionic and covalent compounds? Ionic transfers electrons and covalent shares electrons. When writing a covalent molecule use. Prefixes Do not criss-cross when using...? Covalent Bonds To show the sharing of electrons in a covalent bond you would...? Circle the electrons being shared in each element. Difference Between Ionic and Covalent Compounds Oct 27, 2015 · As their names suggest, ionic compounds are made of ionic bonds, and covalent compounds are made of covalent bonds. Ionic bonds occur between two species which are electrostatically attracted towards each other, whereas covalent atoms bond covalently through the sharing of electrons between their outer shells. Similarities & Differences Between Ionic & Covalent - Sciencing 27 Apr 2018 — For ionic bonds, fixed amounts of ions join together to form an electrically neutral whole with the amounts depending on the excess charges on ...
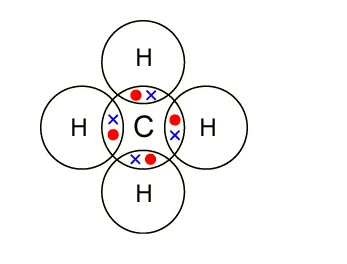
Write 5 points of difference between ionic and covalent ... The ionic bond is the electrostatic force of attraction between two oppositely charged ions. Ionic bonds join metals to non-metals. Examples of ionic bonds are sodium chloride, magnesium chloride, magnesium oxide, etc. Covalent bond: A covalent bond is formed from the mutual sharing of one or more pairs of electrons between two atoms (non-metals).
Difference Between Ionic and Covalent Bonds in Tabular ... The primary difference between ionic and covalent bonds is that an ionic bond is a permanent transfer of valence electrons between two atoms. On the other hand, the covalent bond is sharing of electrons between two atoms. Before going into the deep discussion, let me give you a brief review of the two in a tabular form. ...
Difference Between Covalent and Ionic Bonds - Pediaa.Com Nov 06, 2015 · Covalent bonds and ionic bonds are two different ways of how elements bond to each other. The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other, whereas covalent bonds occur covalently through the sharing of electrons between their outer shells.
Ionic Bonds vs Covalent Bonds | ChemTalk 28 Nov 2021 — Differences between Compounds with Covalent and Ionic Bonds · Ionic bonds tend to transfer electrons, covalent bonds share them more easily ...
Quick Answer: What Are The Similarities And Differences ... The main difference between ionic covalent and metallic bonds is their formation; ionic bonds form when one atom provides electrons to another atom whereas covalent bonds form when two atom shares their valence electrons and metallic bonds form when a variable number of atoms share a variable number of electrons in a Jan 18, 2017.
Differences Between Ionic Covalent and Metallic Bonding ... 10 Differences Between Ionic Covalent and Metallic Bonding 1. Ionic bonding is when metal and non-metal share electrons. 2. Covalent bonding occurs between two nonmetals. 3. Metallic bonds occur when metals share electrons to form a compound. 4. In ionic bonds, the metal has an excess of electrons which it gives up to the non-metal or vice versa.
What are the differences between ionic covalent and ... Ionic bonds result from transfer of electrons, whereas covalent bonds are formed by sharing. 2. Ionic bonds are electrostatic in nature, resulting from that attraction of positive and negative ions that result from the electron transfer process; charge separation between covalently bonded atoms is less extreme.
Difference Between Covalent and Ionic - Difference Wiki Covalent bonds have a low melting and boiling point while Ionic bonds have a higher melting and boiling points. Covalent bonds have low polarity while ionic bonds are more prone to attraction among electrons. There is always a particular structure in covalent bonds while ionic bonds lack any proper shape.
Covalent Bonds vs Ionic Bonds - Difference and Comparison The covalent bond is formed when two atoms are able to share electrons whereas the ionic bond is formed when the "sharing" is so unequal that an electron from atom A is completely lost to atom B, resulting in a pair of ions. Each atom consists of protons, neutrons and electrons. At the centre of the atom, neutrons and protons stay together.
Difference Between Ionic and Covalent Compounds A basic definition of an ionic compound is that they are molecules that consist of charged ions. These ions have opposite (both negative and positive) charges.
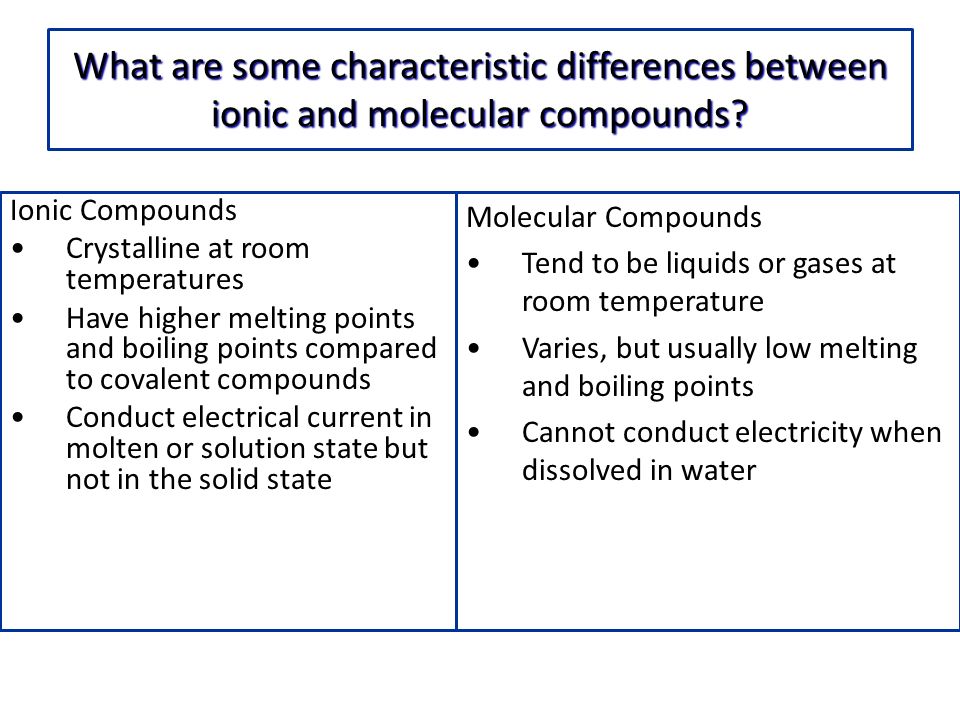
Comparison between Covalent and Ionic Compounds Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons, ionic compounds dissolved in water make an ...
Difference Between Ionic and Covalent Compounds | Compare ... ( Difference Between Ionic and Covalent Bonds ) When ionic bonds are formed, electron (s) is donated by a metal and donated electron (s) is accepted by a non-metal. They form a strong bond due to the electrostatic attraction. Covalent bonds are formed between two non-metals.
Difference Between Ionic Compounds and Covalent Compounds ... The Ionic Compounds exist in the solid-state while Covalent Compounds exist in Solid, Liquid and Gaseous state. Ionic Compounds mostly are soluble in water while the Covalent Compounds are not. Ionic Compounds have very high boiling as well as melting points, while the Covalent Compounds have low melting and boiling points
Main Differences Between Ionic and Covalent Bonds - Examples Since covalent bonds are just sharing their stuff, they aren't as solid of a bond as ionic bonds. You'll also see these as a gas or liquid rather than a solid like ionic bonds. But while covalent bonds might be a little weaker than ionic bonds, they can form between the same elements. The more you know. Difference Between Ionic and Covalent Bonds
What's the difference between ionic and covalent bonds - ZME ... Differences between ionic and covalent bonds. Covalent bonds are much more common in organic chemistry than ionic bonds. In covalent bonds, atoms share electrons, whereas in ionic bonds atoms ...
What is Difference between an ionic bond and a covalent bond? Difference between an ionic bond and a covalent bond Ionic bonding occurs when two atoms have an electronegativity difference greater than 1.7. One of the atoms takes one of the electrons from the outer shell of the other atom, that is, the metallic ion loses an electron, and the non-metallic ion gains the electron.








/covalent-bond-58de6d0b3df78c51627d1783.jpg)











0 Response to "40 difference between ionic and covalent"
Post a Comment