39 how to use a calorimeter
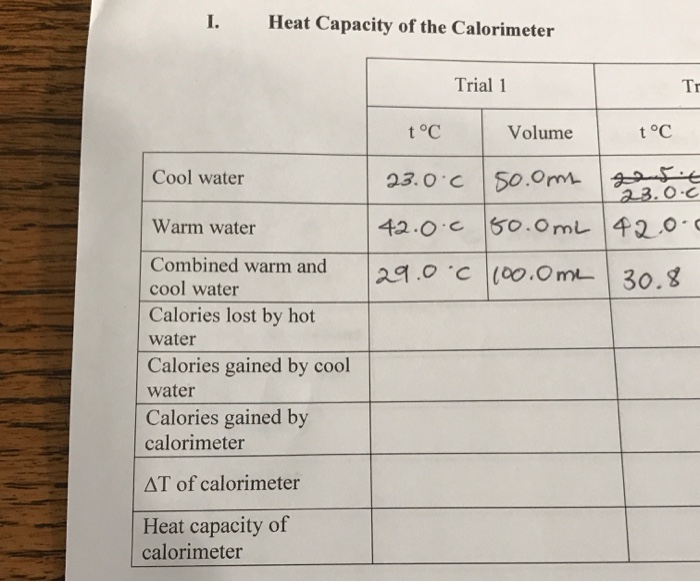
Understanding Calorimetry to Measure Heat Transfer Heat is leaving the water and going into the ice, causing it to melt, so if you watched the temperature on the calorimeter, you'd see the temperature of the water dropping. Eventually, all of the ice would be melted and the water would reach a new state of thermal equilibrium, in which the temperature is no longer changing. Calorimeter to determine the specific heat capacities of liquids Calorimeter to determine the specific heat capacities of liquids Calorimetry deals with the measurement of heat energy.These measurements are based on temperature changes, which are used to determine the amount of heat involved. 1 Test setup 2 Taking heat losses into account 3 Heat capacity of the calorimeter ("water value")
What is a Calorimeter? - Definition, Uses & Equation - Study.com Calorimeters measure the energy transferred between the water in the cup and an object that's placed into the water. Coffee cup calorimeters are often used in high school chemistry calorimetry...

How to use a calorimeter
Differential Scanning Calorimeter (DSC) Market Size In 2022 with Top ... The increasing use of Differential Scanning Calorimeter (DSC) In Drug Analysis, General Chemical analysis, Food Science, Polymers, Metals and other industries is driving the growth of the ... How does a bomb calorimeter work? Combustion Calorimeters measure the heat released from a combustible solid-liquid substance. This is done by weighing a precise measure of the sample substance into a crucible, placing the crucible inside a "bomb" (a sealed metal cylinder called a vessel), filling the vessel with oxygen and igniting the substance. Read More. How to Build a Calorimeter (with Pictures) - wikiHow The formula used to determine the caloric value of a sample of food using a homemade calorimeter is relatively simple: calories = volume of water (in mL) x the temperature change (in Celsius) of the water. 2 Gather the data you need to calculate.
How to use a calorimeter. Coffee Cup Calorimeter - Calculate Enthalpy Change, Constant ... - YouTube This chemistry video tutorial explains how to calculate the enthalpy change using a coffee cup calorimeter at constant pressure. This video contains about 1 practice problem on calorimetry. The... Calorimetry Equation & Calorimeter Uses - Study.com The official equation for calorimetry is {eq}q=mc\Delta t {/eq} In this equation "q" represents the measure of heat transfer. This can be positive or negative. The variable "m" represents the mass... Calorimetry | Chemistry for Non-Majors | | Course Hero In an experiment, 25.0 mL of 1.00 M HCl at 25.0°C is added to 25.0 mL of 1.00 M NaOH at 25.0°C in a foam cup calorimeter. A reaction occurs and the temperature rises to 32.0°C. Calculate the enthalpy change in kJ for this reaction. Assume the densities of the solutions are 1.00 g/mL and that their specific heat is the same as that of water. What Is a Calorimeter and What Is It For? Heat Measurement A calorimeter a device to measure the amount of heat released or absorbed in any physical, chemical or biological process. Modern calorimeters operate in the temperature range of 0.1 to 3500 Kelvin and allow you to measure the amount of heat with an accuracy of .01-10%. The arrangement of calorimeters is very diverse and is determined by the ...
What is a Calorimeter & How Is It Used in a Lab? - Excedr A calorimeter is a device used for calorimetry, or measuring heat capacity or the heat of physical changes or chemical reactions. In pharmaceuticals, they are used in drug design. In the chemical industry, they are used for quality control, and in biological studies, they are used for metabolic rate examination. What Does A Calorimeter Do? How calorimeter are used to analyze fuel How the CAL3K Calorimeter can be used in volatile fuel analysis. As a general rule, volatile fuels are measured very seldom because they are very consistent. However, if the fuel absorbs water, then frequent analysis is called for. Volatile fuel such as alcohol must be prevented from evaporating during the handling process that is from weighing ... So How Exactly Does a Calorimeter Work? - Chemistry | ScienceBriefss.com How do you do a calorimetry lab? Place the thermometer in the calorimeter cup and record the temperature for 3 readings at 30 second intervals. Lift the lid of the calorimeter and drop the pieces of magnesium in, mixing continuously. Record the temperature every 30 seconds until 10 minutes have elapsed. Video advice: CALORIMETRY_Part 01 PDF Calorimetry: Measuring the Energy in Foods - Carolina.com 1. Use a calorimeter to determine the number of calories in 3 samples of food. 2. Construct a model to illustrate the flow of energy through a calorimetry experiment and relate the model to what happens in cells. Next Generation Science Standards* (NGSS) PE HS-LS1-7. Use a model to illustrate that cellular respiration is a chemical process whereby
X-ray astronomy - Wikipedia X-ray astronomy is an observational branch of astronomy which deals with the study of X-ray observation and detection from astronomical objects. X-radiation is absorbed by the Earth's atmosphere, so instruments to detect X-rays must be taken to high altitude by balloons, sounding rockets, and satellites.X-ray astronomy uses a type of space telescope that can see x-ray … Understanding Coffee Cup Calorimetry - Penji Calorimetry is the science of measuring the amount of heat transferred to or from a substance in a reaction by using a calorimeter to measure the heat exchanged with the surroundings. It is important to understand that in calorimetry problems, the substance reacting is the "system" and the water and calorimetry make up the "surroundings". Uses of Calorimeter- Definition, General Principles, Uses, FAQs ... - BYJUS How do you use a colorimeter to measure concentration? Choose the light filter or LED that gives full absorbance. Chart the data in order to get an absorbance graph versus concentration. Then use the instrument to calculate the test solution's absorbance, and use the graph to calculate the solute concentration in the test solution. BYJUS BYJUS
MedGem FDA-Approved Indirect Calorimeter - Metabolic Rate Test The MedGem is an easy-to-use, handheld device that accurately measures oxygen consumption (VO2) to determine resting metabolic rate (RMR)*. ... ♦ MedGem Indirect Calorimeter with a 2-year warranty ♦ One 21-pack Mouthpiece Filters and Nose Clips ♦ MedGem Analyzer Software
eCFR :: 40 CFR Part 1036 -- Control of Emissions from New and In-Use … If you certify with this configuration, you must use 40 CFR 1037.550(a)(3)(ii) to create the vehicle model along with its default transmission shift strategy. Use the transmission parameters defined in Table 1 of § 1036.540 to determine transmission type and gear ratio. For Light and Medium HDVs, use the Light and Medium HDV parameters for the ...
Differential Scanning Calorimeters – TA Instruments Differential Scanning Calorimeters (DSC) measure temperatures and heat flows associated with thermal transitions in a material. Common usage includes investigation, selection, comparison, and end-use performance evaluation of materials in research, quality control and production applications.
Food Calorimetry: How to Measure Calories in Food - Carolina.com Hold the paper clip horizontally and bend the outer end upwards until it is at a 90° angle to the rest of the paper clip. Obtain a 2- to 3-g food sample. Place the food sample on the paper clip's upward-extending end. The sample should be freestanding, supported by the bottom of the paper clip (see Fig. 1).
How to Use Calorimetry in Chemistry - YouTube About the process of calorimetry and how to set up a basic calorimeter.
calorimeter | Definition, Uses, Diagram, & Facts | Britannica calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat capacity of materials. Calorimeters have been designed in great variety. One type in widespread use, called a bomb calorimeter, basically consists of an enclosure in which the reaction takes place, surrounded by a liquid, such as water, that absorbs the heat ...
1.4 Energetics - chemrevise Apr 01, 2020 · calorimeter and not the alcohol Enthalpies of combustion can be calculated by using calorimetry. Generally the fuel is burnt and the flame is used to heat up water in a metal cup. N Goalby chemrevise.org Measuring Enthalpies of Combustion Using Calorimetry • Energy losses from calorimeter • Incomplete combustion of fuel
Use "calorimeter" in a sentence + Audio | The best 66 "calorimeter ... How To Use "calorimeter" In A Sentence? All "calorimeter" example sentences below (+ Audio) are ordered by length from shorter and easier to longer and more complex. To listen to the pronunciation of each sentence, click on button in front of it. (1) Check the calorimeter.
How to use a calorimeter? - MiniScience.com Place the test tube with nails in hot-boiling water for about 10 minutes. This will allow the nails to get to 100ºC temperature without getting wet. Open the lid of the calorimeter and transfer the nails to the inner vessel. Immediately close the lid. Move the stirrer up and down and read the temperature. Record the highest temperature.
Calorimetry | Chemistry | | Course Hero One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). The change in temperature of the measuring part of the calorimeter ...
5.2 Calorimetry - Chemistry Calorimetry is used to measure amounts of heat transferred to or from a substance. To do so, the heat is exchanged with a calibrated object (calorimeter). The change in temperature of the measuring part of the calorimeter is converted into the amount of heat (since the previous calibration was used to establish its heat capacity).
Dynamic Mechanical Analyzers – TA Instruments Dynamic Mechanical Analysis measures the mechanical properties of materials as a function of time, temperature, and frequency. In addition to basic material properties, DMA also quantifies finished part characteristics, reflecting the important contribution that processing has on …
Calorimetry - measuring energy changes from combustion Calorimetry - measuring energy changes from combustion Energy can be released in chemical reactions as light, sound or electrical energy. But it is most often released as heat energy. Measuring...
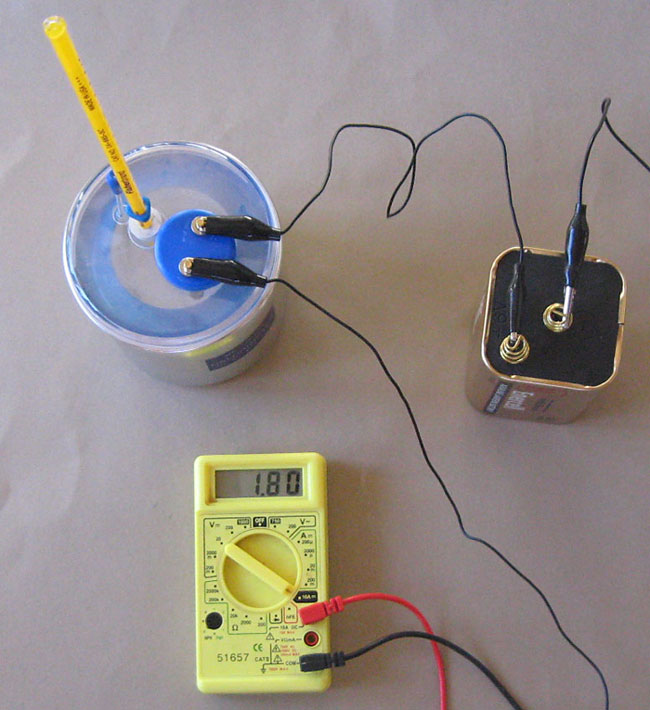
What is a Calorimeter Used For? (Video) - Mometrix A calorimeter is an instrument for which we know (or can determine) the heat capacity, which means we can calculate the heat released by a sample by measuring the temperature change of the calorimeter using this equation. q calorimeter = c calorimeter × m a s s c a l o r i m e t e r × Δ T c a l o r i m e t e r
How a calorimeter can be used to determine the identity of an unknown ... While the water/metal mixture is boiling, record the mass of the empty calorimeter cup. You can identify an unknown substance by measuring its density and comparing your result to a list of known densities. Density = mass/volume. Assume that you have to identify an unknown metal. You can determine the mass of the metal on a scale.
How to Build a Calorimeter (with Pictures) - wikiHow The formula used to determine the caloric value of a sample of food using a homemade calorimeter is relatively simple: calories = volume of water (in mL) x the temperature change (in Celsius) of the water. 2 Gather the data you need to calculate.
How does a bomb calorimeter work? Combustion Calorimeters measure the heat released from a combustible solid-liquid substance. This is done by weighing a precise measure of the sample substance into a crucible, placing the crucible inside a "bomb" (a sealed metal cylinder called a vessel), filling the vessel with oxygen and igniting the substance. Read More.










0 Response to "39 how to use a calorimeter"
Post a Comment