40 difference between ionic and molecular compounds
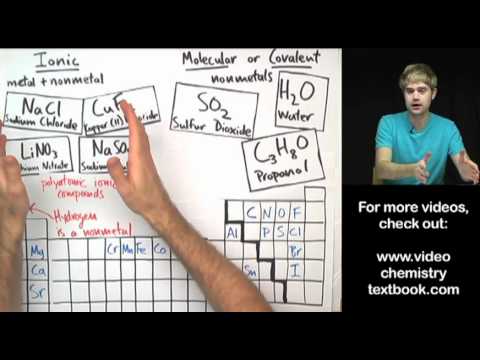
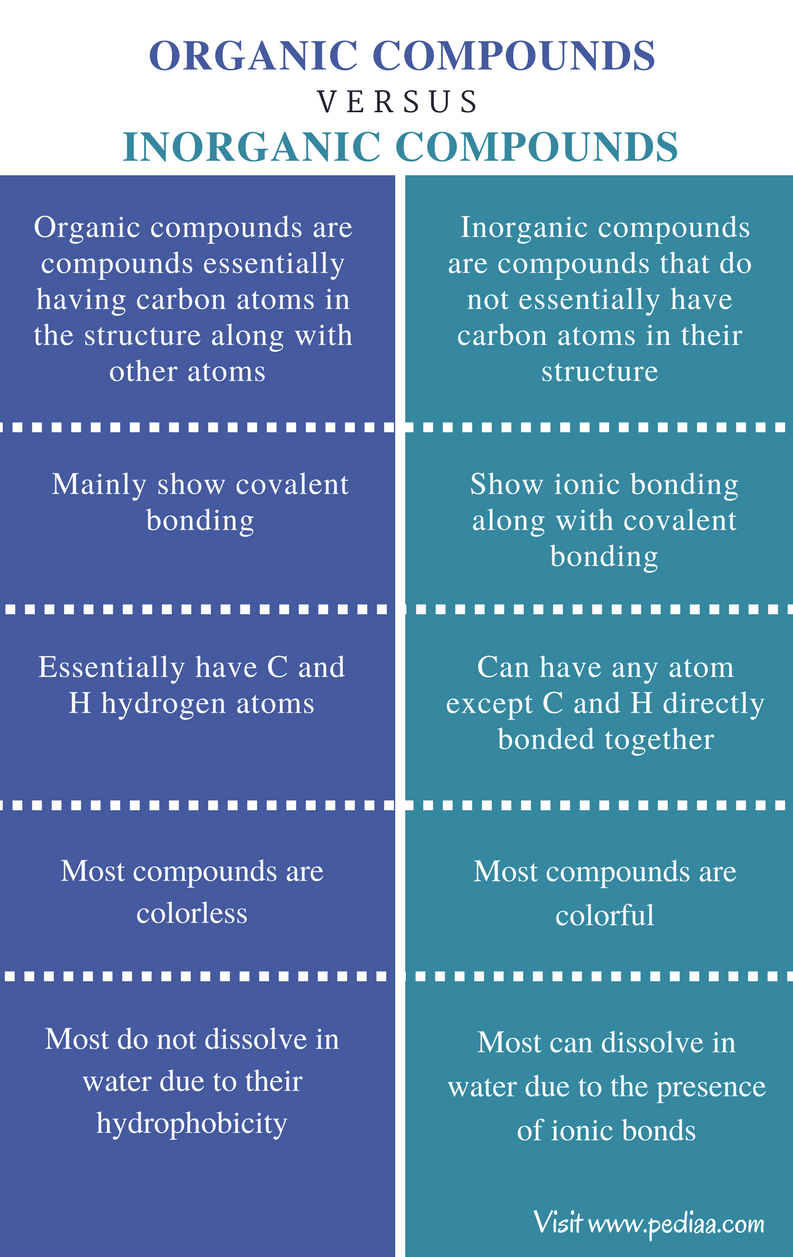
Difference Between Ionic and Covalent Compounds - Pediaa.Com Almost all the compounds in Chemistry can be broadly categorized into Ionic and Covalent Compounds. They differ from each other due to the bonding type between the atoms that take part in making a molecule/ compound. As their names suggest, ionic compounds are made of ionic bonds, and covalent compounds are made of covalent bonds. Difference between Ionic and Molecular Compound The ionic and molecular compounds are two types of compounds that are present in the solid state. The difference between them is that the ions present in the ionic compounds are held together by electrostatic forces of attraction. The compounds are formed by the combination of metal and non-metal atoms.
Ionic Compounds Vs. Molecular Compounds: What You Need to Know Ionic compounds are charged ions, whereas molecular compounds consist of molecules. A molecular compound cannot conduct electricity in any state, whereas an ionic compound, when dissolved in an aqueous solution, can act as a good conductor of electricity. Ionic compounds are more reactive than molecular compounds.

Difference between ionic and molecular compounds
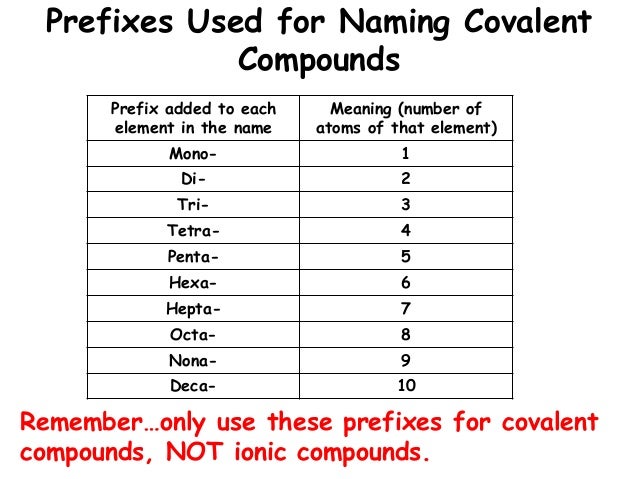
Difference Between Ionic and Molecular Compound Molecular compounds are pure substances formed when atoms are linked together by sharing of electrons while ionic compounds are formed due to the transfer of electrons. 2. Molecular compounds are made due to covalent bonding while ionic compounds are made due to ionic bonding. 3. Difference Between Ionic and Molecular Compound - STEPBYSTEP Molecular Compounds Molecular compounds are formed when two or more neutral atoms, i.e. having similar number of protons and electrons, join together by sharing the electron or electrons in their outer-most shell. The form of bonding between these atoms is known as covalent bonding. Molecular compounds are formed between two or more non-metals. Difference Between Ionic vs Molecular Compounds - Whatisdiff They are good conductors of electric force. Ionic compounds are represented in a formula unit. They have high water solubility, which means they can easily dissolve in water. The bonding method is by covalent sharing, where electrons transfer between elements ionically. Definition of Molecular Compounds
Difference between ionic and molecular compounds. Ionic vs. Molecular - YouTube To see all my Chemistry videos, check outhttp://socratic.org/chemistryHow can you tell the difference between compounds that are ionic and molecular (also kn... What Is The Main Difference Between Ionic Bond And Covalent Bond What are the four main differences between covalent bonding and ionic bonding? Covalent bonds are formed between two non-metals, whereas ionic bonds are formed between a metal and non-metal. Molecules formed by covalent bonds have a low melting point, whereas those with ionic bonds have a high melting point. Difference Between Ionic and Molecular Solids Key Difference - Ionic vs Molecular Solids. Solid substances are compounds that exist in a solid state at a given temperature and pressure. Solid state means, the atoms, molecules or ions in that substance are tightly packed, avoiding the movement of those chemical species (unlike in liquids or gases). What is the difference between molecular compound and ionic compound ... 3. Molecular compounds are formed between two non-metals whereas ionic compounds are formed between metals and non-metals. 4. Molecular compounds are poor conductors of electricity while ionic compounds are good conductors of electricity. 5. Molecular compounds can be found in any physical state '" solid, liquid, or gas. Ionic compounds are ...
Ionic and Molecular Compounds: Structures & Properties To solve this question, you need to know what makes a compound ionic or molecular. We said before that ionic compounds consist of a cation and an anion, whereas molecular compounds possess covalent bonds. Cu (NO 3) 2 is an ionic compound because Cu 2+ is a cation, and NO 3- is a polyatomic anion known as carbonate. What's the difference between an ionic and molecular compound? Molecular compounds are pure substances formed when atoms are linked together by sharing of electrons while ionic compounds are formed due to the transfer of electrons. … Molecular compounds are formed between two non-metals while ionic compounds are formed between metals and non-metals. Are ionic or covalent bonds more common? Difference Between Ionic and Molecular Compounds The key difference between ionic and molecular compounds is that the ionic compounds have electrostatic attraction forces between cations and anions whereas the molecular compounds have only covalent chemical bonds between the atoms. Chemical elements can join with each other to form chemical compounds. Difference Between Ionic and Molecular Compound - UrbanPro Molecular compounds are formed between two non-metals while ionic compounds are formed between metals and non-metals. 4. Molecular compounds are poor electrical conductors while ionic compounds are good conductors. 5. Molecular compounds can be in any physical state '" solid, liquid, or gas. Ionic compounds are always solid and crystalline ...
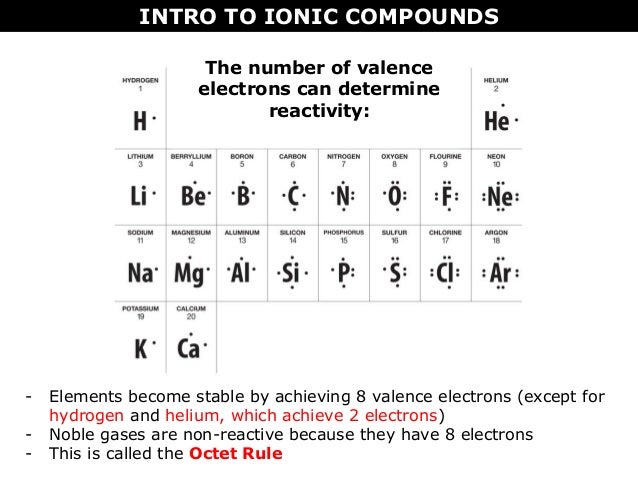
What is the difference between Ionic and Molecular compounds They are usually between a metal and a nonmetal or a polyatomic ion. Ionic compounds are bonded with the cation "giving away" its valence electrons and the anion "receiving" them for a full octet. Molecular compounds are usually between two or more nonmetals. The nonmetals simply share their valence electrons for a full octet. Difference Between Ionic Compounds and Molecular Compounds The main difference between Ionic and Molecular Compounds is that the electrons of atoms in ionic compounds are transferred between the elements because of the presence of a difference in electronegativity. However, in the case of molecular compounds, the electrons are only shared but not transferred. 10 Differences Between Ionic and Molecular Compound 2022 Update The most important difference between ionic and molecular compounds lies in their formation. Ionic compounds are formed by the transfer of electrons while molecular compounds are formed by the sharing of electrons. Molecular compounds are also called covalent compounds. Ionic compounds have ionic bonds and molecular compounds have covalent bonds. Difference between ionic and molecular compounds - LORECENTRAL The ionic compounds are linked by ionic bonds, while the molecular compounds are covalently bonded. An ionic bond is formed between two oppositely charged ions by means of electrostatic attraction. By Coulomb's law, the force of an electrostatic attraction is based on the proximity of atoms and the force of the electric charge.
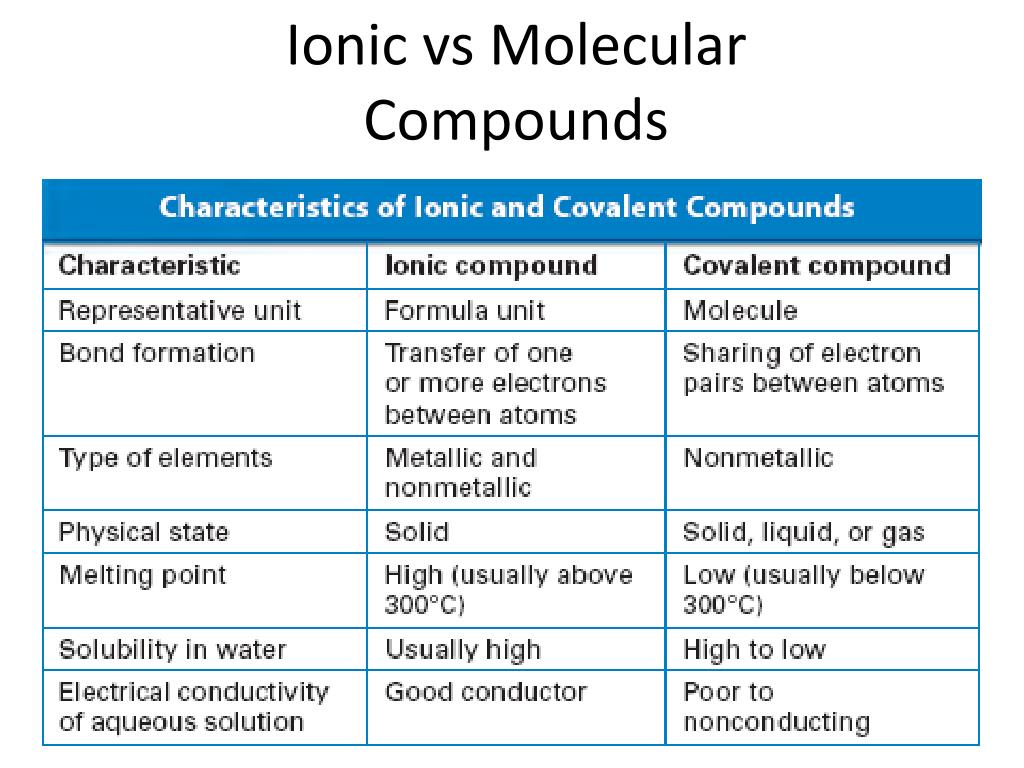
PDF Differences between Ionic and Molecular compounds 7. Ionic compounds do not have ; discrete units. They have an extended . array of alternating positive and negative ions, their formula is the simplest . ratio of these ions, also called formula . unit. Their basic unit is a discrete molecule ; i.e. why they are called molecular . compounds and their formula is called . molecular formula.
Difference Between Ionic and Molecular Compounds Ionic compounds are made of ionic bonds where the atoms are electrostatically attracted towards each other. Molecular compounds are made of covalent bonds where the electrons are shared between the atoms involved in the formation. Species involved Ionic compounds occur through the interaction between cations and anions.
What's the difference between ionic compounds and molecular ... - Quora Answer (1 of 4): The most important difference between ionic and molecular compounds lies in their formation. Ionic compounds are formed by the transfer of electrons while molecular compounds are formed by the sharing of electrons. Molecular compounds are also called covalent compounds. Ionic com...
What Is the Difference Between Ionic and Molecular Compounds? Ionic compounds form through the transfer of electrons, while molecular compounds form as a result of electron sharing. Ionic compounds contain atoms and molecules with opposite charges. They bond together as a result of their opposite charges. For instance, a negatively paired ion bonds with a positive ion.
Differences between ionic and molecular compounds - Quizlet Memorize flashcards and build a practice test to quiz yourself before your exam. Start studying the Differences between ionic and molecular compounds flashcards containing study terms like Ionic, Covalent, Ionic and more.
Difference Between Ionic Compounds and Molecular Compounds Ionic compounds are formed due to electrostatic force of attraction between metals and non-metals, whereas, Molecular Compounds are formed due to sharing of electrons between two non-metals. Molecular Compounds are formed when two non-metals chemically combine, on the other hand, Ionic Compounds are formed between metal and non-mental.
Ionic vs Covalent Bonds - Understand the Difference Updated on January 23, 2020 A molecule or compound is made when two or more atoms form a chemical bond, linking them together. The two types of bonds are ionic bonds and covalent bonds. The distinction between them has to do with how equally the atoms participating in the bond share their electrons. Ionic Bonds
Difference between Ionic and Molecular Compound Ionic compounds tend to be brittle and have high melting and boiling points, while molecular compounds are usually softer and have lower melting and boiling points. Ionic compounds also conduct electricity when dissolved in water, but molecular compounds do not.


0 Response to "40 difference between ionic and molecular compounds"
Post a Comment