40 catalase and hydrogen peroxide reaction
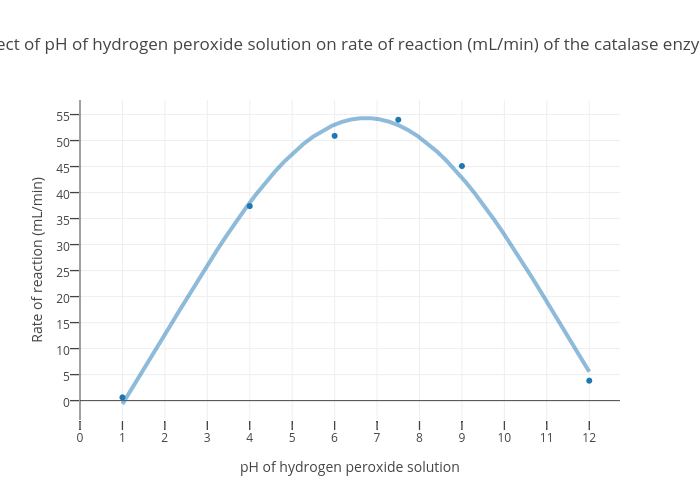
Concentrations of hydrogen peroxide VerkkoNEVER use 35% hydrogen peroxide externally on people, other animals or plants. NEVER use 35% hydrogen peroxide internally for people or other animals. All home applications of 35% hydrogen peroxide require a great amount of dilution. Note: As of this writing (June 2018) Amazon does not sell concentrations of peroxide higher that … practicalbiology.org › bio-molecules › factorsInvestigating an enzyme-controlled reaction: catalase and ... Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Cells make the enzyme catalase to remove hydrogen peroxide. This investigation looks at the rate of oxygen production by the catalase in pureed potato as the concentration of hydrogen peroxide varies. The oxygen produced in 30 seconds is collected over water.
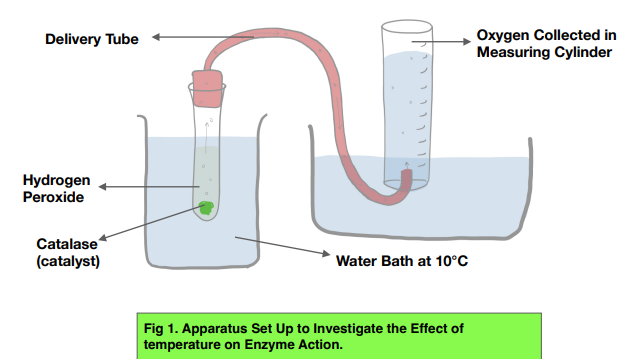
› science-fair › articleCatalase and Hydrogen Peroxide Experiment - Education Using a potato and hydrogen peroxide, we can observe how enzymes like catalase work to perform decomposition, or the breaking down, of other substances. Catalase works to speed up the decomposition of hydrogen peroxide into oxygen and water. We will also test how this process is affected by changes in the temperature of the potato.
Catalase and hydrogen peroxide reaction
Is Hydrogen Peroxide A Substrate Of Catalase? - On Secret Hunt Hydrogen peroxide bubbles when it comes into contact with an enzyme called catalase. Most cells in the body contain catalase, so when the tissue is damaged, the enzyme is released and becomes available to react with the peroxide. … The bubbles you see when you pour hydrogen peroxide on a cut are bubbles of oxygen gas. Why is catalase in potatoes? Hydrogen Peroxide And Catalase Reaction Lab - 491 Words | Bartleby The hypothesis was tested by adding 50ml of hydrogen peroxide (H2O2) into four different concentrations of Catalase (25%, 50%, 75%, and 100%). The temperature of the Catalase was measured before and 30 seconds after the hydrogen peroxide (H2O2) had been added. It was discovered that the rate of reaction initially increased as the concentration ... About The Equation For The Catalase Reaction With Hydrogen Peroxide ... In this case, enzyme catalse has a specific active site, which just for the substrate hydrogen peroxide to fit in, then the reaction takes place. The reaction involved is hydrolysis. At low temperature, the reaction takes place very slowly, this because molecules are moving relatively slowly as have low kinetic energy.
Catalase and hydrogen peroxide reaction. The effect of catalase on hydrogen peroxide - EduCheer! Hydrogen peroxide is converted into two harmless substances, oxygen and water with the help of the enzyme catalase, which speeds up the reaction. The more substrate molecules present, the more collisions happen, and more enzyme activity until all the active sites are full causing the reaction to slow down. The more enzymes present the reaction ... Reaction of catalase with hydrogen peroxide - Essay examples database Reaction of catalase with hydrogen peroxide AIM: I seek to find the speed of effect between catalase and hydrogen peroxide. Digestive enzymes such as Catalase are healthy proteins molecules which have been found in living cells. They can be used to improve specific reactions in the cellular material. Reaction of Catalase and Hydrogen Peroxide - YouTube It catalyzes the decomposition of hydrogen peroxide to water and oxygen. Function: (Catalase) + 2 H2O2 → 2 H2O + O2 The presence of catalase in a microbial or tissue sample can be... Difference Between Catalase and Peroxidase The key difference between catalase and peroxidase is that catalase catalyzes the decomposition of hydrogen peroxide into water and oxygen, whereas peroxidase catalyzes the decomposition of peroxides. Therefore, catalase is a type of peroxidase enzyme.
Reaction Between Catalase and Hydrogen Peroxide - Nature Reaction of Catalase and Hydrogen Peroxide - YouTube Students describe how an enzyme works in both cold water and warm water. You can see a clear reaction in both instances, but much more bubbling in the warm t... Why Does Hydrogen Peroxide Fizz On Cuts? | Live Science Verkko23.2.2011 · Hydrogen peroxide (H2O2), a compound made up of two hydrogen atoms and two oxygen atoms, begins to breaks apart as soon as it contacts blood, creating that stinging sizzle. Reaction between Catalase and Hydrogen Peroxide | Nature IN a recent communication in Nature1, Dr. P. George described manometric experiments which showed a rapid initial decay of the enzyme compared with which the well-known decay due to irreversible ...
Hydrogen peroxide Reactions and Physical Properties | H2O2 Hydrogen peroxide Reactions, Physical Properties an Uses| H 2 O 2. Hydrogen peroxide is a very common chemical compound in the laboratory and have lot of uses in home and industrial scale. It is used as a disinfectant in the cleaning of injuries. In this tutorial, we will learn about preparation, reactions and other characteristics of hydrogen ... How does catalase break down hydrogen peroxide? - UC Santa Barbara Answer 1: In our body the enzyme catalase catalyses the reaction 2H2O2 = 2H2O + O2, the decomposition of hydrogen peroxide into water and oxygen. So, how does catalase work? The protein has a certain 3D structure when it is active, which contains a channel into which the hydrogen peroxide can diffuse. Catalase And Hydrogen Peroxide Reaction Lab - 1378 Words | Bartleby Because of this, hydrogen peroxide in cells must be regulated and this is done by catalase. Catalase is an enzyme that catalyzes the dissociation of hydrogen peroxide into water and oxygen gas, preventing the formation of oxidizing hydroxide radicals (Yang et al. 2002). It does this by binding to the hydrogen peroxide. Get Access Hydrogen peroxide - Wikipedia VerkkoHydrogen peroxide is a chemical compound with the formula H 2 O 2.In its pure form, it is a very pale blue liquid that is slightly more viscous than water.It is used as an oxidizer, bleaching agent, and antiseptic, usually as a dilute solution (3%–6% by weight) in water for consumer use, and in higher concentrations for industrial use.. Concentrated …
Catalase−Peroxidase Activity of Iron(III)−TAML Activators of Hydrogen ... Exceptionally high peroxidase-like and catalase-like activities of iron(III)−TAML activators of H2O2 (1: Tetra-Amidato-Macrocyclic-Ligand FeIII complexes [Fe ... Catalytic Oxidation of Trypan Blue Using Copper Complexes and Hydrogen Peroxide Shows a Negative Reaction Order. Industrial & Engineering Chemistry Research 2021, 60 (4) ...
Why does catalase react violently with hydrogen peroxide but ... - Quora The decomposition of hydrogen peroxide yields water and oxygen gas. Now, the thing is, Thermodynamics tells us that the reaction can occur energywise (because of its negative values of ∆H and ∆G); however, Chemical kinetics prevents its from happening at a noticeable rate.
What type of reaction is catalase and hydrogen peroxide? What happens when you mix catalase and hydrogen peroxide? Vigorously, it gives out O2 gas 2H2O2 → 2H2O + O2↑ *Catalase ,an enzyme which can act as a catalyst helps dissociate hydrogen peroxide! *It has heme group that is active in this catalysis. *This enzyme releases O2 with considerable response
Catalase - Wikipedia VerkkoCatalase is used in the food industry for removing hydrogen peroxide from milk prior to cheese production. Another use is in food wrappers, where it prevents food from oxidizing. Catalase is also used in the textile industry, removing hydrogen peroxide from fabrics to make sure the material is peroxide-free.. A minor use is in contact lens hygiene – a …
Catalase | Function & Applications | Britannica Found extensively in organisms that live in the presence of oxygen, catalase prevents the accumulation of and protects cellular organelles and tissues from damage by peroxide, which is continuously produced by numerous metabolic reactions. In mammals, catalase is found predominantly in the liver. Catalase has various industrial applications.
Reaction of Catalase with Hydrogen Peroxide - Beowulf Essays Reaction of catalase with hydrogen peroxide AIM: I aim to find the rate of reaction between catalase and hydrogen peroxide. Enzymes such as Catalase are protein molecules that are found in living cells. They are used to speed up specific reactions in the cells. Each enzyme just performs one particular reaction so they are all very specific.
Investigating an enzyme-controlled reaction: catalase and hydrogen ... VerkkoClass practical or demonstration. Hydrogen peroxide (H 2 O 2) is a by-product of respiration and is made in all living cells. Hydrogen peroxide is harmful and must be removed as soon as it is produced in the cell. Cells make the enzyme catalase to remove hydrogen peroxide.. This investigation looks at the rate of oxygen production by the …
Catalase Substrate, Function & Enzyme - Study.com Catalase is an extremely efficient enzyme with the ability to break down millions of hydrogen peroxide molecules per second. Its four identical subunits allow it to process multiple reactions at ...
Effect of Enzyme Catalase on Hydrogen Peroxide - UKEssays.com Hydrogen peroxide is the by-product of respiration. As an oxidizer, it will decompose to form oxygen and water. The chemical equation for the decomposition of hydrogen peroxide is 2 H2O2 → 2 H2O + O2. The reaction is speeded up by the presence of enzyme, namely catalase which is used in this experiment.
Hydrogen Peroxide: the body's best defence system Verkko18.1.2017 · This reaction of hydrogen peroxide with hemoglobin is also the basis of the “luminol” test used by crime scene investigators to detect traces of blood that may not be visible at all. ... To protect itself, the body makes catalase, the enzyme that decomposes hydrogen peroxide before it can form hydroxyl radicals.
Food Grade Hydrogen Peroxide Benefits - Earth Clinic VerkkoFood grade hydrogen peroxide can come in strengths varying from 3% to 35%. So you'd have to use 10-12 drops of 3% before you'd get to the same level as one drop of 35%. Hydrogen peroxide is a general stimulant to metabolic rate. Low metabolic rate is a pro-viral influence, so H2O2 might show antiviral effects.
Hydrogen Peroxide Therapy: Benefits and Side Effects - Earth Clinic VerkkoHydrogen peroxide, or H202 as it is scientifically known, comes in a variety of forms. Depending on the concentration of the mixture, the liquid is considered for household use, food grade, or electrical uses. In whatever form, though, hydrogen peroxide is nothing more than oxygen and water combined in a unique ratio to form a germicidal liquid.
Enzymes in Action - Catalase - Southern Biological To combat the ever-present threat of hydrogen peroxide, living cells produce an enzyme known as Catalase that rapidly breaks hydrogen peroxide down into water and oxygen. This experiment, studies the effect of substrate concentration on the rate of reaction.
Catalase - Wikipedia Catalase can also catalyze the oxidation, by hydrogen peroxide, of various metabolites and toxins, including formaldehyde, formic acid, phenols, acetaldehyde and alcohols. It does so according to the following reaction: H 2 O 2 + H 2 R → 2H 2 O + R The exact mechanism of this reaction is not known.
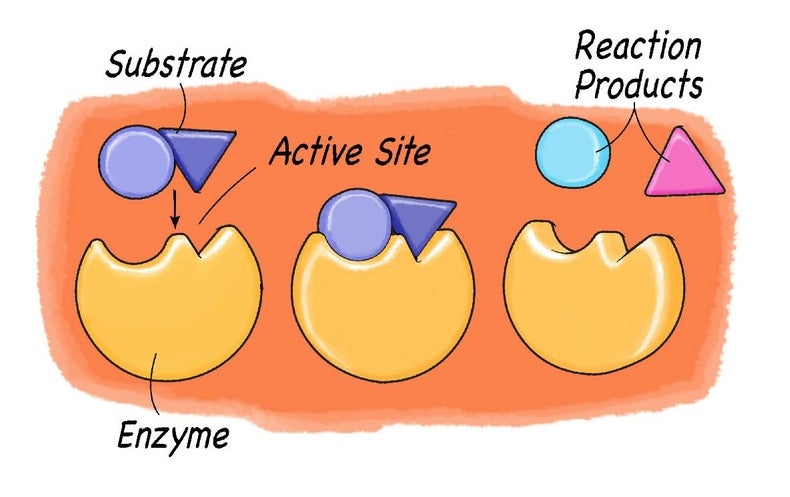
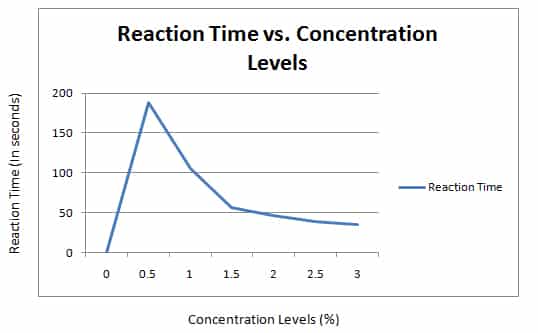
Effect of Substrate Concentration on the Rate of Activity of Catalase It catalyses the decomposition of hydrogen peroxide into water and oxygen. 2H 2 O 2 + Catalase >>> 2H 2 O + O 2 Catalase dramatically reduces the activation energy needed for the reaction. Without catalase, the decomposition would take much longer and would not be fast enough to sustain human life.
Reaction between catalase and hydrogen peroxide - PubMed Reaction between catalase and hydrogen peroxide. ... Reaction between catalase and hydrogen peroxide Nature. 1947 Jul 12;159(4054):41-3. doi: 10.1038/160041a0. Author P GEORGE. PMID: 20252563 DOI: 10.1038/160041a0 No abstract available. MeSH terms Catalase* ...
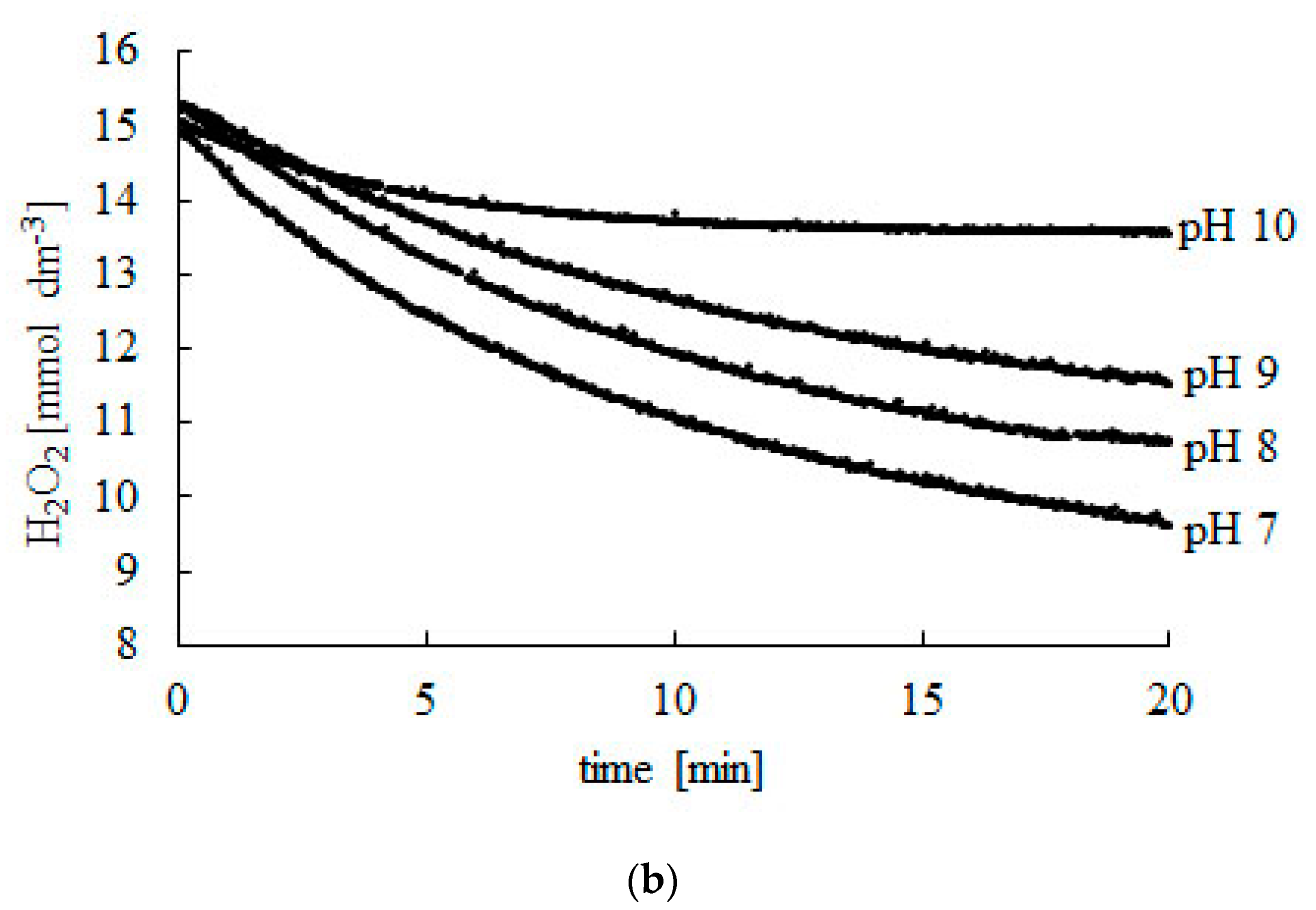
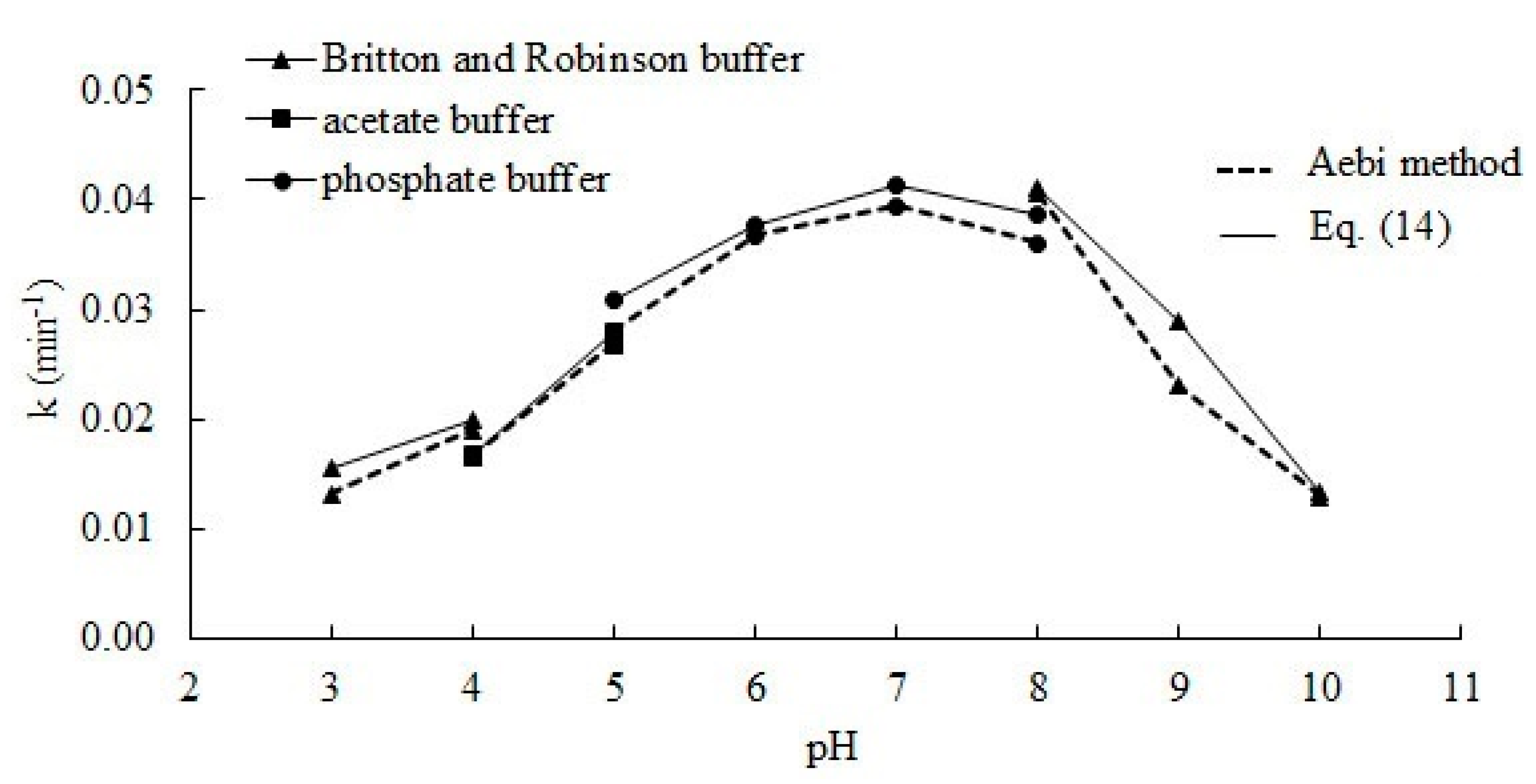
The Decomposition of Hydrogen Peroxide by Liver Catalase - Pmc It is concluded that the reaction involves primarily adsorption of hydrogen peroxide at the catalase surface. 2. The decomposition of hydrogen peroxide by catalase is regarded as involving two reactions, namely, the catalytic decomposition of hydrogen peroxide, which is a maximum at the optimum pH 6.8 to 7.0, and the "induced inactivation" of ...
Catalase | Function & Applications | Britannica Verkkocatalase, an enzyme that brings about (catalyzes) the reaction by which hydrogen peroxide is decomposed to water and oxygen. Found extensively in organisms that live in the presence of oxygen, catalase prevents the accumulation of and protects cellular organelles and tissues from damage by peroxide, which is continuously produced by …
The molecular mechanism of the catalase reaction - PubMed Catalases are ubiquitous enzymes that prevent cell oxidative damage by degrading hydrogen peroxide to water and oxygen (2H (2)O (2) --> 2 H (2)O + O (2)) with high efficiency.
[Catalase: structure, properties, functions] - PubMed VerkkoCatalase (EC 1.11.1.6) ... If the concentration of H2O2 is high, catalase acts catalytically, i.e. removes H2O2 by forming H2O and O2 (catalatic reaction). However, at a low concentration of H2O2 and in the presence of a suitable hydrogen donor, ... Hydrogen Peroxide / metabolism
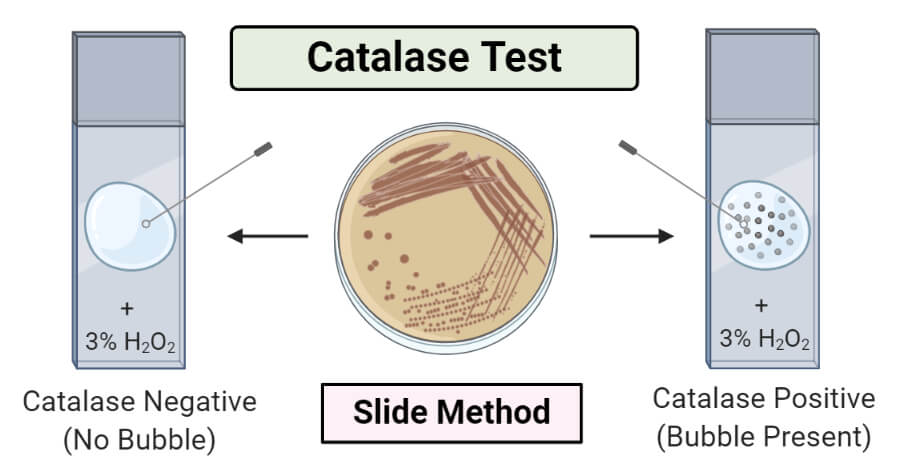
Catalase Enzyme - Chemical Nature, Functions and Important FAQs - VEDANTU Catalase Enzyme Reaction Catalase \[2H_{2}O_{2} \rightarrow H_{2}O + O_{2}\] The activity of catalase can be illustrated by taking a microbial sample and adding hydrogen peroxide to it. Bubble formation in the reaction indicates the release of oxygen. The activity of the enzyme is so rapid that it can be observed with naked eyes.
About The Equation For The Catalase Reaction With Hydrogen Peroxide ... In this case, enzyme catalse has a specific active site, which just for the substrate hydrogen peroxide to fit in, then the reaction takes place. The reaction involved is hydrolysis. At low temperature, the reaction takes place very slowly, this because molecules are moving relatively slowly as have low kinetic energy.
Hydrogen Peroxide And Catalase Reaction Lab - 491 Words | Bartleby The hypothesis was tested by adding 50ml of hydrogen peroxide (H2O2) into four different concentrations of Catalase (25%, 50%, 75%, and 100%). The temperature of the Catalase was measured before and 30 seconds after the hydrogen peroxide (H2O2) had been added. It was discovered that the rate of reaction initially increased as the concentration ...
Is Hydrogen Peroxide A Substrate Of Catalase? - On Secret Hunt Hydrogen peroxide bubbles when it comes into contact with an enzyme called catalase. Most cells in the body contain catalase, so when the tissue is damaged, the enzyme is released and becomes available to react with the peroxide. … The bubbles you see when you pour hydrogen peroxide on a cut are bubbles of oxygen gas. Why is catalase in potatoes?









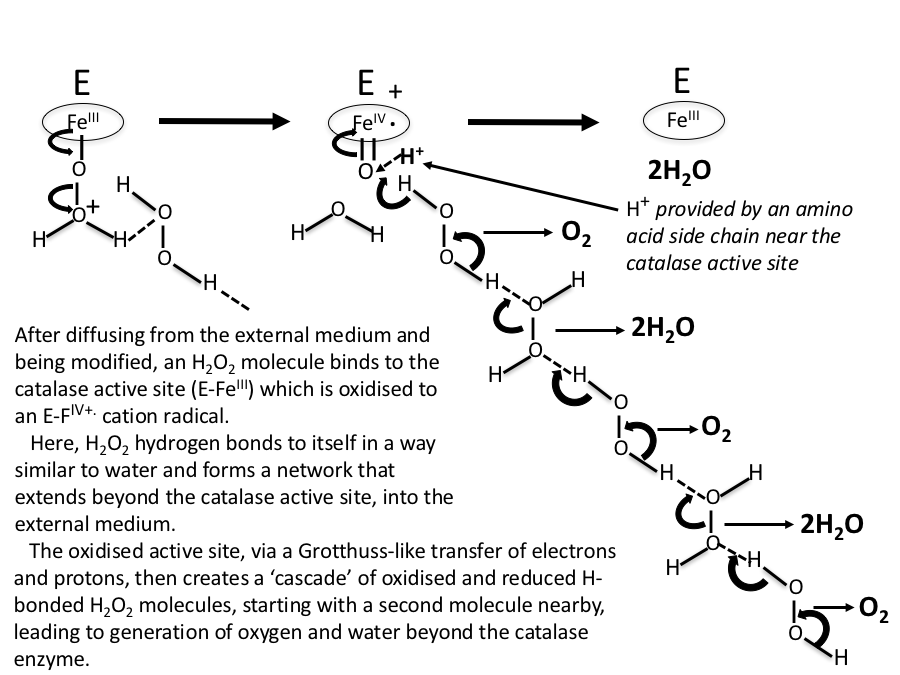
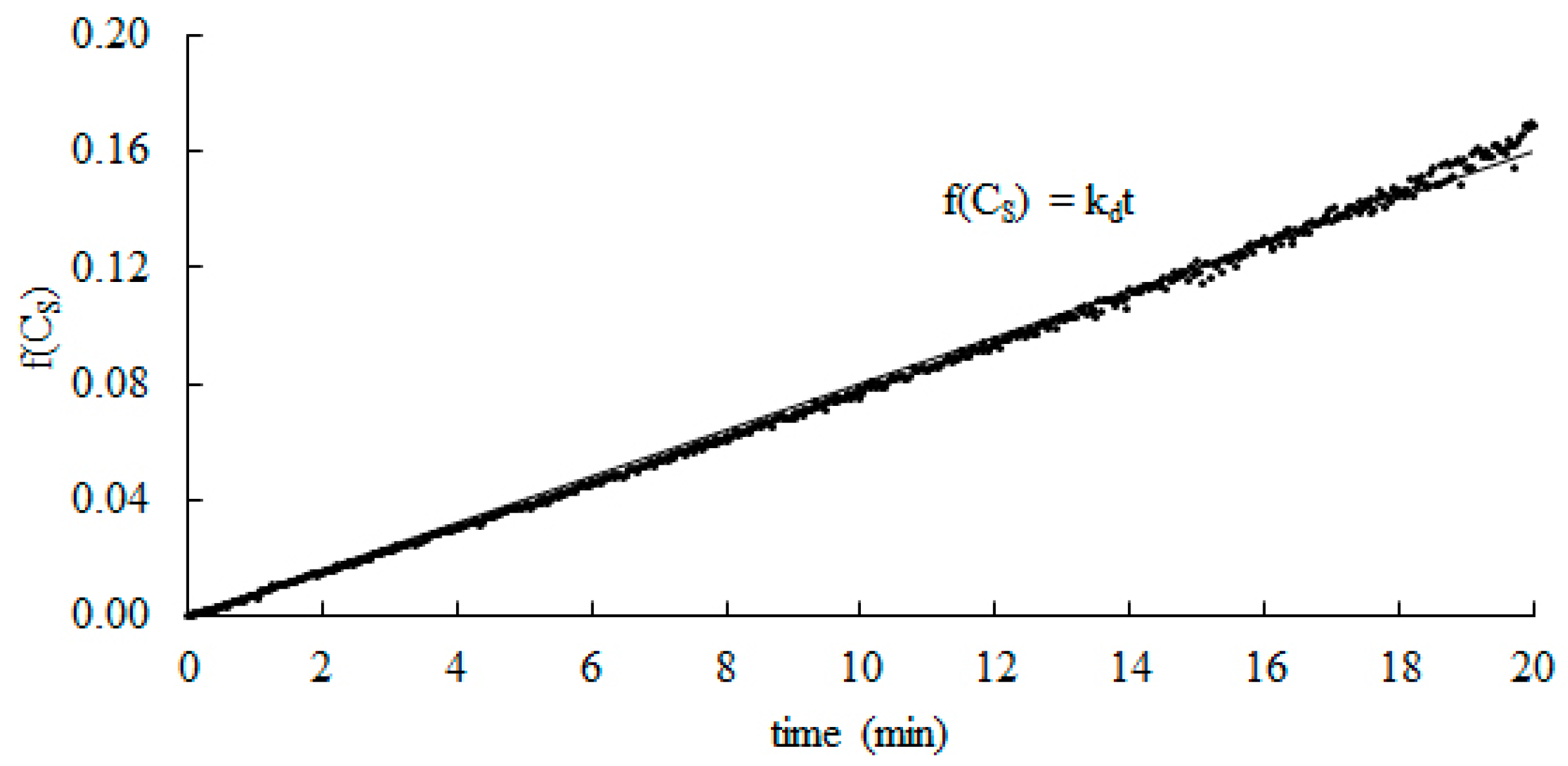

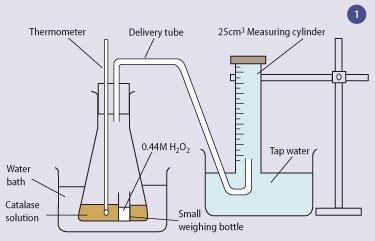










0 Response to "40 catalase and hydrogen peroxide reaction"
Post a Comment